Page 15 - 2019_01-Haematologica-web
P. 15
Editorials
Migliaccio Laboratory to play a critical role in the develop- ment not only of thrombosis but also progression to myelofibrosis in a GATA1low mouse model.11 In this animal model, abnormal localization of P-selectin in both megakaryocytes and platelets led to increased platelet/leukocyte interactions, an increased incidence of thrombotic events, and increased release of neutrophil pro- teases and transforming growth factor-beta which plays a critical role in the development of bone marrow fibrosis, osteosclerosis and disease progression in MPN. Importantly, the progression to MF as well as the increased frequency of thromboses in the GATA1low mice was not observed in mice in which P-selectin was deleted by genetic approaches.12,13 The role of integrins in MPN thrombosis has been further supported by the provocative work of Edelmann et al.14 who showed that JAK2V617F+ granulocytes
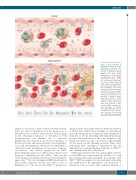
Figure 1. The mechanism of thrombus formation in myelo- proliferative neoplasm. The Jak2V617F mutation causes an increase in endothelial cell (EC) Weibel-Palade body (WPB) degranulation of P-selectin and von Willebrand factor (VWF); translocation of Rap1 towards the cell membrane with activa- tion of the integrins LFA1 and VLA4; and increased neutrophil extracellular trap (NET) forma- tion. In addition, a red blood cell-platelet interaction through FasL/FasR causes externaliza- tion of phosphatidylserine (PS). All of these events play a role in thrombus formation. Rap1: Ras-related protein 1; LFA1: lymphocyte function-associated antigen 1; STAT: signal trans- ducer and activator of tran- scription; PAD4: peptidyl argi- nine deimidase 4; ICAM-1: intracellular adhesion molecule 1; VCAM-1: vascular cell adhe- sion molecule 1; VLA4: very late antigen-4: FasL: Fas ligand.
and monocytes were characterized by increased activation of VLA-4 and/or LFA1. These integrins are cell adhesion molecules which play an essential role in the attachment of leukocytes to EC by interacting with intracellular matrix proteins. The translocation of these two integrins to the granulocyte surface was due to the effects of mutated JAK2 on the inside–outside signaling molecule, Rap1. Most importantly these investigators demonstrated that the administration of integrin-blocking antibodies to JAK2V617F+ mice diminished the rate of thrombosis.
Additional evidence for the role of neutrophils in throm- bosis in MPN was recently offered by Wolach et al.15 with their demonstration that neutrophils from patients with JAK2V617F MPN are primed to form neutrophil extracellular trap, implicated in the pathogenesis and promotion of thrombosis. Moreover, mice with conditional knock-in of
haematologica | 2019; 104(1)
5