Page 178 - 2018_12-Haematologica-web
P. 178
P.L.R. Nicolson et al.
tion coefficient 0.959). Inhibition of whole cell phospho- rylation and phosphorylation of Syk Y525/6, SLP-76 Y145, Btk Y551 and LAT Y200 was seen at a 7 mM dose of ibru- tinib which is 10-fold higher than the maximal concentra- tion in patients (Figure 3Bii, 3Biii). The IC50 values for each phosphorylation event, when they could be calculated, are included in Online Supplementary Table S1. Blockade of Btk pY223 and PLCg2 phosphorylation by 70 nM ibrutinib was also observed with lower concentrations of CRP or in the absence of the integrin αIIbβ3 blocker, eptifibatide (Online Supplementary Figures S2Aiv and S2B). There was no significant increase in phosphorylation of PLCg2 up to 180 s in response to CRP in the presence of 70 nM ibruti- nib (Online Supplementary Figure S2Ci-iii).
Due to the absence of phosphospecific antibodies for the Btk-related Tec family kinase, Tec, the effect of ibruti- nib on Tec phosphorylation was investigated following immunoprecipitation and re-probing with the antiphos- photyrosine monoclonal antibody 4G10. The effect of ibrutinib was biphasic with partial blockade at 170 nM and full blockade observed at 7 mM (Figure 3C).
These results demonstrate that a concentration of 70 nM ibrutinib is sufficient to block Btk at its autophospho- rylation site and on PLCg2 on Y753, Y759 and Y1217 and
that this effect is irreversible. At this concentration of ibru- tinib, aggregation in response to a high concentration of CRP is delayed but is not blocked. At a 10- to 20-fold high- er concentration, ibrutinib reversibly blocks aggregation in parallel with reversible loss of phosphorylation of Src on Y418. Reversible inhibition of tyrosine phosphorylation of other proteins is seen at a 100-fold higher concentration than that required for blockade of Btk. Ibrutinib causes biphasic inhibition of phosphorylation of Tec, with inhibi- tion occurring at 3- to 5-fold higher concentrations than those required to block phosphorylation of Btk on Y223, and full blockade at a 100-fold higher concentration. These results are consistent with loss of Tec autophospho- rylation at 170 nM of ibrutinib and loss of phosphoryla- tion on the activation site by higher concentrations.
Low-dose ibrutinib has no effect on platelet adhesion and aggregation in response to collagen under flow conditions
The relevance of the observation that aggregation is delayed but not blocked in response to high concentra- tions of CRP and collagen was addressed using flow stud- ies in which GPVI functions in conjunction with other tyrosine kinase-linked receptors that also signal via Btk,
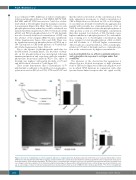
Ai
Aii
Bi
Bii
Figure 4. A low dose of ibrutinib has no effect on platelet adhesion to collagen under flow. (A) Washed platelets (10x108/mL) were incubated with ibrutinib or vehicle (DMSO) for 5 min and stimulated with CRP (10 mg/mL). (i) Representative trace and (ii) mean data from three identical experiments show a characteristic delay associ- ated with inhibition of Btk autophosphorylation. (B) Platelets were reconstituted with autologous red blood cells and platelet-poor plasma and flowed at arterial shear over collagen-coated microcapillaries for 3 min before being fixed and imaged. (i) Representative differential interference contrast images are shown. (ii) Platelet cover- age as a percentage of values for vehicle-treated platelets was calculated and is shown as mean ± SEM from three identical experiments. *P<0.05, ns: non-significant.
2102
haematologica | 2018; 103(12)