Page 14 - 2018_12-Haematologica-web
P. 14
Editorials
tion of Runx1 and C/EBPα. In mature myeloid cells, Twist2 negatively regulates pro-inflammatory responses by inhibit- ing the expression of pro-inflammatory cytokines such as interleukin-12, interferon-g, interleukin-1, tumor necrosis factor-α, interleukin-6, and macrophage inflammatory pro- tein-1α, through the inactivation of c/EBPα and NF-kB. According to these data, Twist1 and Twist2 play opposite roles in myeloid differentiation although how the opposing functions of these two homologs in myeloid differentiation are balanced remains unclear.
Twist1 in acute myeloid leukemia
Twist1 plays an important role in the process of epithe- lial-mesenchymal transition. The overexpression of Twist1 has been described as a poor prognostic factor in numerous epithelial-derived malignancies such as breast cancer, prostate cancer, colorectal cancer, bladder cancer,
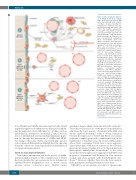
Figure 1. Effect of inducible deficien- cy of Twist1 in the bone marrow niche. (A) Normal bone marrow (BM) niche. The normal BM niche constists of multiple cell types, including non- hematopoietic endothelial cells (EC), CXCL12-expressing adventitial cells (CAR cells), Leptin-receptor-positive cells (LepR+ cells), Nestin – green flu- orescent protein high-expressing cells [Nestin-GFP(high) cells], myelinated fibers (surrounded by Schwann cells) and non-myelinated sympathetic fibers; mesenchymal stem cells (MSC); osteoblasts; and adipocytes; and hematopoietic cells that include regulatory T cells (Treg), macrophages (Mac) and osteoclasts (OC). The mechanisms used by these cells to control HSC activity include expres- sion of short-acting hormones, cytokines and chemokines, leptin, stem cell factor (SCF); angiopoietin; thrombopoietin (TPO); C-X-C motif chemokine 12 (CXCL12); transform- ing growth factor-beta (TGFβ); neuro- transmitters (norepinephrine and neu- ropeptide Y) and purine nucleotides (ATP and adenosine); extracellular proteins [osteopontin (OPN), fibronectin, and others] and direct cell-to-cell, contact-dependent interac- tions (e.g. CXCL12-CXCR4; integrin- VCAM1) and transfer of organelles and secondary messengers. (B) The effect of a Twist1-deficient BM niche on HSC. Twist1 deficiency in BM niche causes EC expansion and angiogene- sis, depletion of MSC, increased OPN production and decreased intramar- row levels of CXCL12, VCAM1 and SCF production while OPN production increases, which impairs HSC reten- tion in the BM niche and promotes their mobilization to the periphery. (C) The effect of a Twist1-deficient BM niche on leukemic stem cells (LSC). The Twist1-deficient BM niche pro- motes acute myelogenous leukemia (AML) after transformation by the MLL-AF9 oncogene. The mechanism involves upregulated Notch signaling through upregulation of the produc- tion of local Jagged-2 (expressed by EC, osteoblasts and MSC) and the membrane expression of Notch recep- tors on LSC.
melanoma, hepatocellular carcinoma and neck carcinoma.7 Twist1 has been found to be overexpressed in mononuclear cells from the bone marrow of patients with AML and chronic myeloid leukemia,12 with a strong correlation between the expression of Twist1 and Bmi-1, an essential polycomb complex group with a fundamental role in the maintenance of leukemia stem cells. In fact, AML patients whose blasts overexpress Twist1 have a more aggressive clinical phenotype, with a good response to the cell cycle phase-specific agent cytarabine but not to the non-cell cycle phase-specific anthracycline, daunorubicin.13 It also been shown that Twist1 expression is augmented in the HSC and progenitor compartment and decreased in bone marrow stromal cells from patients with myelodysplastic syn- drome.14 However, whether Twist1 in the bone marrow niche participates in AML pathogenesis is not clear.
MLL-AF9 is an oncoprotein, a product of chromosome
1938
haematologica | 2018; 103(12)