Page 110 - 2018_11-Haematologica-web
P. 110
J. Chlebowska-Tuz et al.
AC
B
D
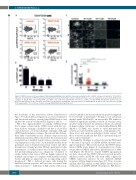
Figure 5. SK053 decreases the percentage of KG1 leukemia-initiating cells and their clonogenic potential in vitro. (A) KG1 cells were incubated for 72 h with the indicated concentrations of SK053, harvested and stained for flow cytometry. Representative density plots from a series of experiments are presented. (B) Mean per- centages of CD123+ KG1 cells among CD34+ and CD38– cells ± SD (n=3 experiments) *P<0.05 vs. controls, one-way ANOVA with Dunnett post-hoc test. (C) Representative pictures of the clonogenic assay plates on day 14 after seeding KG1 cells pretreated for 72 h with SK053. (D) Mean clone size (pixel area) ± SEM (n=3 experiments); **P<0.0001 vs. controls, one-way ANOVA with Dunnett post-hoc test.
the feasibility of this interaction (Online Supplementary Figure S10) and further precipitation, as well as enzymatic and functional analyses, revealed that SK053 binds to and inhibits the activity of human PDI (Figures 2 and 3).
PDI is the original member of a family of PDI proteins that contain a characteristic CXXC motif, with two cys- teine residues forming a disulfide bond that is cleaved by oxidoreductases or by thiol-disulfide exchange. Although all PDI family members contain a thioredoxin-like domain, they differ considerably in size, domain composition and enzymatic properties. PDI is involved in the formation and isomerization of disulfide bonds between cysteine residues of polypeptides as they fold.19 Such disulfide modifications participate in post-translational protein control and affect the functions of many proteins. PDI also participates in the maintenance of cellular homeostasis by mediating oxida- tive protein folding and acts as a chaperone.19 A number of studies indicate that various PDI are highly expressed in multiple cancer types as compared with matched normal tissues and play an important role in supporting cancer progression.19 PDI is induced in tumor cells during the unfolded protein response and has been associated with chemoresistance.20,21 Inhibition of PDI activity with a non-
selective inhibitor, bacitracin, enhanced apoptosis triggered by bortezomib or fenretinide.22 Propynoic acid carbamoyl methyl amide (PACMA31), an irreversible PDI inhibitor, exhibited significant antitumor effects in ovarian cancer models23 and potentiated the antitumor effects of sorafenib in hepatocellular carcinoma.24 A structurally different PDI inhibitor (CCF642) was shown to exert anti-myeloma activity associated with the induction of endoplasmic retic- ulum stress and apoptosis-inducing calcium release.25 All these observations indicate that PDI is a potential drug tar- get in cancer treatment.
Remarkably, Heafliger et al. have shown that PDI binds to the stem loop region of C/EBPa mRNA, thereby block- ing its translation.13 Our results indicate that SK053 upreg- ulates C/EBPa levels (Figure 4), downregulates its down- stream effector SOX4, and induces differentiation of AML cells (Figure 1F). However, despite C/EBPa upregulation we have observed that SK053 still exerts potent growth- inhibitory effects in HL-60 cells with stably suppressed CEBPA expression induced by two different shRNA sequences (Online Supplementary Figure S7). Thus, it seems unlikely that PDI inhibition with SK053 directly leads to stabilization of CEBPA mRNA. Nonetheless, PDI knock-
1850
haematologica | 2018; 103(11)