Page 172 - 2018_09-Mondo
P. 172
J. Qiao et al.
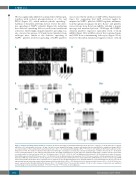
3B) was significantly inhibited on immobilized fibrinogen, together with reduced phosphorylation of c-Src and PLCγ2 (Figure 3C), which mediate platelet spreading.5,7 Addition of thrombin (2 U/mL) did not reverse the defec- tive spreading of NLRP3-/- platelets (Figure 3A), indicating that defective spreading did not result from insufficient activation. Interestingly, impaired platelet spreading was also observed in apyrase (1 U/mL)-treated platelets from wild-type mice, similar to the defective spreading of NLRP3-/- platelets. Defective spreading of NLRP3-/-platelets
was rescued by the addition of ADP (Online Supplementary Figure S2), suggesting that ADP secretion might be impaired in NLRP3-/- platelets. NLRP3 regulates inflamma- tion through processing pro-IL-1β to IL-1β,27 and platelets excise introns from IL-1β pre-mRNA, yielding a mature message and translated protein.28 Wild-type and NLRP3- deficient platelets expressed equivalent levels of IL-1β mRNA (Figure 3D-i) in RNA extracts that contained mini- mal RNA from contaminating leukocytes (Figure 3D-ii); however, thrombin stimulation triggered release of IL-1β
AB
C D(i) D(ii)
E(i) E(ii) F
Figure 3. Platelet spreading and interleukin-1β secretion. (A) Platelet spreading on immobilized fibrinogen in the presence or absence of 10 ng/mL IL-1β, 0.5 μg/mL anti-IL-1β or 2 U/mL thrombin. Scale bar = 20 μm. Covered area was quantified by Image J software and analyzed by one-way ANOVA for comparison. Images (X100) are representative of three independent experiments (mean ± SD, n = 3). Compared with wild-type (WT), **P<0.01; ns: not significant. (B) Platelet adhesion on fib- rinogen for 90 min. The number of platelets that adhered to fibrinogen-coated glass coverslips was calculated using Image J software (mean ± SE, n = 3) (Student t-test). (C) Phosphorylation of c-Src and PLCγ2 in platelets after spreading on fibrinogen for 90 min. Data were quantified using Image J software and are represented as a ratio relative to total level (mean ± SD, n = 3) (Student t-test). *P<0.05, **P<0.01. (D) Total RNA was isolated from 5 x 108/mL platelets in order to measure IL-1β mRNA expression by RT-PCR (mean ± SD, n = 3) (Student t-test) (i); Washed platelet preparations (5 x 108/mL) were found to contain approximately 2 x 104/mL leukocytes. RNA was isolated from 5 x 108/mL platelets or 2 x 104/mL leukocytes followed by reverse transcription into cDNA which was used to measure IL-1β mRNA expression by PCR. PCR products were evaluated on 1.5% agarose gel (ii). (E) Washed platelets were stimulated with 0.5 U/mL thrombin before the level of IL-1β in supernatants was measured by enzyme-linked immunosorbent assay (mean ± SE, n = 3-5) (Student t-test) (i) and western blot (mean ± SD, n = 3) (ii). (F) Active cas- pase-1 expression after thrombin stimulation (mean ± SD, n = 3) (Student t-test). The western blot analysis of IL-1β or active caspase-1 expression was quantified as fold change to the level without treatment. *P<0.05;**P<0.01; ***P<0.001. ns: not significant.
1572
haematologica | 2018; 103(9)