Page 100 - 2018_09-Mondo
P. 100
B.C. Ede et al.
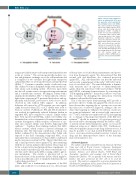
Figure 6. Proposed mechanism of action of parthenolide (PTL) and the protective effect provided by mesenchymal stem cells (MSC). PTL causes apoptosis by increas- ing reactive oxygen species (ROS) stress and decreasing reduced glutathione (rGSH) resulting in death of T-cell acute lymphoblas- tic leukemia (T-ALL) cells (A). MSC express high levels of the cystine glutamate antiporter xc-, facilitat- ed by the antiporter protein xCT. Extracellular cystine is taken up by MSC and reduced into cysteine. Cysteine is released into the extra- cellular space for uptake by T-ALL cells, blocking ROS induction and preventing apoptosis (B). Blocking the xCT system using sul- fasalazine (SSZ) or siRNA reduces protective effects provided by MSC (C).
using a specialized amino acid transport mechanism known as the xc– system.14 The system specifically mediates cys- tine and glutamate exchange across the cell membrane and is comprised of two subunits: the light chain transporter subunit SLC7A11 (or xCT) and the heavy subunit SLC3A2 (4F2hc).30,31 Cysteine is unstable outside of cells and is rap- idly oxidized to form a disulphide bridge with another cys- teine amino acid, forming cystine. However, upon entry into the cell, cystine enters a stronger reducing environment and is converted into cysteine. Blocking xc– activity with a small molecule inhibitor, SSZ,32 or by knockdown of the xc– light chain component xCT, prevented thiol release and sig- nificantly reduced MSC protection to levels close to those observed in cells without MSC support. In addition, leukemia cell survival in a PTL resistant case was signifi- cantly decreased when SSZ or xCT siRNA were used to block xc– activity. Furthermore, PTL and SSZ treatment in vivo resulted in significantly reduced leukemia burden in engrafted NSG mice compared to controls. Similar results have been reported in BCP-ALL, where cell viability was diminished in a subset of patients following treatment with cysteine dioxygenase, which catalyzes conversion of cys- teine into cysteine sulfinic acid, thereby bypassing GSH synthesis.33 Together, these findings indicate that MSC release cysteine, which confers a survival advantage on leukemia cells. The functional contribution of thiols was further confirmed by the demonstration that T-ALL cells exposed to the thiol containing compound NAC, at an equivalent thiol concentration to that in MSC conditioned media, were more resistant to PTL. The fact that there was still a small protective effect against PTL after interfering with the xc- system, indicates that cysteine release may not be the sole protective mechanism in these experiments. A recent study found that ALL cells release extracellular vesi- cles that can be taken up by MSC, causing a shift to glycol- ysis metabolism. The switch to glycolysis led to an increased release of the metabolite lactate, which might be used as an additional source of energy by leukemia cells and confer chemoresistance.34
While MSC are a fundamental component of the BM niche, and contribute to its formation in vivo, other stromal
cells may have a role in leukemia maintenance and protec- tion from therapeutic agents. We demonstrated that BM stromal cells and fibroblasts also conferred protection against PTL. ALL cells themselves can alter the endosteal and vascular compartments of the niche12 and induce apop- tosis of osteoblast cells.17 The remodelled niche is dynami- cally transient and on exposure to chemotherapeutic agents, ALL cells can release CCL3 and cytokines TGF-β1 and GDF15, conferring chemoresistance by activating the TGF-β signaling pathway.12 It may be possible to overcome this resistance by disrupting the interactions between leukemia cells and the BM environment.
This is the first report demonstrating MSC provide a protective effect to T-ALL cells against PTL. Moreover, we have shown that targeting the xc- system can overcome this effect (see overview in Figure 6), adding to the evi- dence that targeting xc– enhances the efficacy of anti-can- cer agents in leukemias9 and in several solid cancer mod- els.35-38 MSC viability was unaffected by PTL and targeting xc-, so damage to this important element of the BM envi- ronment39,40 should be minimal. A logical progression of this work will be determining whether the effects of PTL, or indeed current chemotherapeutic agents, in vivo can be enhanced using an xc- inhibitor like SSZ. Furthermore, with developments in nanoscopic drug delivery vectors, it may be possible to use dual loaded (PTL+SSZ) nanovec- tors to overcome selective patient resistance towards one drug and the chemoprotective effects of the leukemia microenvironment in vivo.
Acknowledgments
The authors would like to thank Dr Jeremy Hancock, Mr Paul Virgo and staff of Bristol Genetics Laboratory, Southmead Hospital for excellent technical assistance. We also thank Elinor Curnow, Statistics and Clinical Studies, NHS Blood and Transplant, consultants and oncology staff at Bristol Royal Hospital for Children. We are grateful to the patients and their families who gave permission for their cells to be used for research. This work was supported by a generous donation from Mr Richard Cunningham and by grants from the Department of Health and NHS Blood and Transplant.
1500
haematologica | 2018; 103(9)