Page 78 - Haematologica August 2018
P. 78
1314
G.J. Roboz et al. AB
CD
as predicted. There are several possible reasons for this, including: a) suboptimal dose and/or schedule of plerix- afor, leading to inadequate CXCR4 blockade; b) subopti- mal CXCR4 blockade despite adequate dosing; c) ade- quate CXCR4 blockade, but presence of additional factors influencing the dependency of LSCs on their microenvi- ronment, such as VLA-4 and/or E-selectin. Also, CXCR4- expressing LSCs were more effectively mobilized than those without CXCR4 expression and plerixafor effective- ly increased the cycling of stem/progenitor cells, but we did not measure the effect of increased cycling on the abil- ity of decitabine to incorporate into these cells. Our data show that leukemia stem and progenitor cells persist after treatment with decitabine. Persistence of LSCs has been associated with disease progression in larger AML studies using chemotherapy,16 but the significance of phenotypi- cally-defined LSCs in disease progression or clinical out-
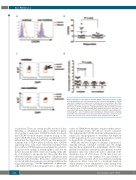
Figure 5. Plerixafor is more likely to mobilize CXCR4+ cells and increase the cycling of stem/progenitor cells. (A) Representative flow cytometry histograms for CXCR4 expression in diagnostic samples from a mobilizer and a non-mobilizer. Blue filled histogram represents control, red represents CXCR4-stained. (B) Scatter plots for the expression of CXCR4 at diagnosis comparing mobilizers and non-mobilizers within the responder group. (C) Representative flow cytometry dot plots to determine cell cycle status. Red squares show quiescent cells. (D) Scatter plots representing Ki-67+ stem/progenitor cells in mobilizers and non-mobilizers during plerixafor-containing cycles (PD) and non-plerixafor cycles (D). Each symbol represents a patient, horizon- tal bar represents the mean, error bars represent the Standard Error of Mean.
comes for HMA-based therapies requires further investi- gation in larger studies. We did not observe consistent data indicating that CXCR4 blockade with plerixafor sen- sitizes LSCs and progenitors to decitabine, but depletion of LSC populations by cycle 3 among responders seemed to be predictive of longer remission duration.
While this trial was designed to optimize acquisition of clinically relevant, correlative scientific data, the design may not have optimized the antileukemic effects of the plerixafor/decitabine combination, since patients received plerixafor only every other cycle and for only half of the decitabine doses (5 of 10 doses). At the trial outset, we were uncertain as to the optimal dose and schedule of plerixafor, and concerned about its potential for inducing leukocytosis. These issues led to a treatment design in which plerixafor was administered either during even numbered or odd-numbered cycles for each dosing
haematologica | 2018; 103(8)