Page 77 - Haematologica August 2018
P. 77
Decitabine and plerixafor in elderly AML
Phenotypically defined LSCs were evaluated in the bone marrow during treatment. It is clear that LSCs were shown to persist even after multiple cycles of decitabine (Figure 6). An increase in LSC frequency was noted after cycle 3, when relapses were beginning to become evident (Figure 6A). Interestingly, among non-mobilizers, there was a continuous increase in LSCs (Figure 6B), suggesting the inability of decitabine to eliminate these cells. Furthermore, among responders, the absence of an increased population of LSCs after cycle 3 correlated with prolonged remission, with relapse in most patients only after 8 cycles of therapy. In contrast, all patients with a greater than two-fold increase in LSCs at cycle 3 relative to the previous bone marrow time point replapsed before completing 7 cycles of therapy (Figure 6C).
Discussion
This investigator-initiated clinical trial was the first time a mobilizing agent has been combined with a DNA methyltransferase inhibitor in newly diagnosed patients with AML. The study was based on the hypothesis that
AB
blockade of CXCR4/SDF-1 signaling with plerixafor would mobilize leukemic stem and progenitor cells out of their bone marrow microenvironment and make them more susceptible to the effects of decitabine. We found that adding plerixafor to decitabine was safe and tolerable. The toxicity profile was as expected for older patients with newly diagnosed AML treated with 10-day cycles of decitabine and included myelosuppression, febrile neu- tropenia, and infections. Importantly, the addition of pler- ixafor did not induce clinically significant hyperleukocyto- sis or tumor lysis syndrome. Grade 1 and 2 insomnia and gastrointestinal disturbances were more frequent with plerixafor, but there were no major, unexpected events. We established the maximum tolerated dose of plerixafor as 810 μg/kg and the recommended treatment dose for combination with decitabine as 675 μg/kg, based on an event of renal insufficiency, combined with increased episodes of gastrointestinal complaints and insomnia.
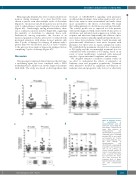
We designed extensive correlative scientific studies in an effort to understand the effects of plerixafor on leukemic stem and progenitor populations. Treatment with plerixafor resulted in significant mobilization of leukemic stem and progenitor cells, but not as effectively
C
D
Figure 4. Plerixafor increas- es mobilization of stem/progenitor cells. (A) Violin plot representing the fold change, evaluated at 4 hours after infusion, in stem/progenitor cells (CD34+CD38-) for all patients comparing cycles containing plerixafor (DP) to cycles with- out plerixafor (P). Scatter plots representing the aver- age fold change in stem/progenitor cells (CD34+CD38-) comparing (B) responders and non-repon- ders in all cohorts, (C) cohorts A and B, and (D) all cohorts. Each symbol repre- sents a patient, horizontal bar represents the mean, error bars represent the Standard Error of Mean. Paired t-tests between plerix- afor and non-plerixafor cycles.
haematologica | 2018; 103(8)
1313