Page 41 - Haematologica June
P. 41
potential changes of HSC contribution to hematopoiesis independent of their numbers (Figure 4D). In line with our observations following primary transplantation, JQ1- treated HSC also contributed more to hematopoiesis in secondary recipients, indicating a sustained long-term effect of JQ1 on HSC. The contribution of DMSO-treated HSC to hematopoiesis decreased in secondary recipients over the course of 12 months in agreement with literature.22 Interestingly, the decline was slower upon transplantation of JQ1-treated HSC, and after 12 months they still significantly contributed to hematopoiesis while any contribution of DMSO-treated HSC to the host hematopoiesis was no longer detectable (Figure 4D). Therefore, the relative contribution of JQ1 over DMSO- treated HSC to hematopoiesis increased over time, indi- cating a sustained long-term effect of JQ1 on HSC (1 month: 1.9-fold, 3 months: 3.4-fold, 6 months: 5.2-fold, 12 months: 11.8-fold). Furthermore, the JQ1-induced increase in HSC proliferation did not translate into enhanced exhaustion of HSC, as there was no significant difference
in long-term survival of secondary recipients from both groups (Figure 4E).
JQ1 treatment accelerates blood recovery after sublethal irradiation
As JQ1 treatment increases HSC numbers by inducing proliferation, we asked whether the pro-stem-cell pheno- type elicited by JQ1 might translate into increased hematopoietic recovery after sublethal irradiation, or if this beneficial effect is compromised by increased radiosensitiv- ity of HSC due to increased cell cycle activity.
To verify this, mice were treated with JQ1 for three weeks before they received sublethal irradiation and hematopoietic recovery was monitored. As we found that JQ1 also medi- ates HSC expansion in 11-week old mice that have been treated with JQ1 for three weeks (Online Supplementary Figure S2I), we used adult mice for these experiments.
Analysis of the BM of irradiated mice revealed a strong decrease in hematopoietic stem and progenitor cells with- in two weeks after irradiation (Figure 5A and B).
JQ1 induces proliferation of HSC
ABCD
EFG
HI
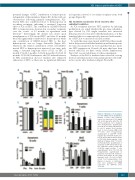
Figure 2. JQ1 induces T-cell apopto- sis and inhibits B-cell maturation. Animals received daily intraperi- toneal (i.p.) injections of 50 mg/kg JQ1 for 21 days after which the dif- ferent parameters were analyzed. (A-C) Flowcytometric quantification of Pro-B (A), Pre-B (B) and immature B cells to analyze B-cell develop- ment in bone marrow (BM) (n=4; *P<0.05). (D-F) Flowcytometric quantification of CD4–CD8– double negative (D), CD4+CD8+ double posi- tive (E) and all differentiation states of CD4–CD8– double negative T cells (DN1-4) in thymus (n=4; *P<0.05). (G) Flowcytometric quantification of apoptosis in CD4–CD8– double nega- tive, CD4+CD8+ double positive and CD4/CD8 single positive T cells using Annexin V (n=3; *P<0.05). (H) Flowcytometric quantification of apoptosis in pro-B, pre-B, immature- B (Imm) and mature B cells using Annexin V (n=4; *P<0.05). (I) FACS- sorted T-cell subpopulations from thymus of placebo or JQ1-treated mice were analyzed for mRNA expression via qRT-PCR (n=5; *P<0.05).
haematologica | 2018; 103(6)
943