Page 130 - Haematologica June
P. 130
1032
D. Drandi et al.
previously identified by Sanger sequencing.1 Whereas the ASqPCR standard curve confirmed the reported sensitivi- ty of 1.00x10-3 (0.25% out of 6000 WT),6 ddPCR reached a 1.5 log higher sensitivity (0.0035% out of 30000 WT) also because of the larger amount of gDNA used in ddPCR than in ASqPCR (100 ng vs. 20 ng) (Figure 2 and Online Supplementary Table S2). Overall, 100 WM samples (48 BM, 52 PB, 40 baseline and 60 follow up) from 48 patients, as well as 20 control samples (15 healthy subjects and 5 with multiple myeloma) were tested by both methods (Online Supplementary Figure S3). An example of mutation analysis performed by both methods on 2 patients is pre- sented in Online Supplementary Figure S4. Of the 40 base- line samples tested by both methods, 35 (87.5%) scored positive and 4 (10%) scored negative for MYD88L265P by both ddPCR and ASqPCR (with there being only one dis- cordant ddPCR+/ASqPCR– case), while higher number of discordances were observed among follow-up samples: 13/60 (21.7%) ddPCR+/ASqPCR–, and 11/60 (18.3%) ddPCR–/ASqPCR+ (Online Supplementary Table S3). Indeed, the strength of agreement was very good in the baseline cases (Cohen κ=0.8) but poor in follow-up samples (Cohen κ=0.2), resembling what had been previously shown for low burden infiltrated samples.31 All control samples scored negative by both methods (median: ddPCR 1.75x10-4 (range 3.10x10-4 – 2.70x10-5) and ASqPCR DCT=10 (range DCT=8.4-10.7, setting DCT=8 as the cut- off for negativity).
Comparison of MYD88L265P and IGH-based digital droplet polymerase chain reaction assays for the purposes of minimal residual disease detection
To investigate whether ddPCR of MYD88L265P could be used for MRD detection, we compared it to the highly sensitive IGH-based MRD ddPCR assay. Only patients with available follow-up samples were screened for IGH rearrangements. A clonal VDJ rearrangement was detect- ed in 34/52 (65%) patients, as expected in WM.32 All these patients scored positive for MYD88L265P by ddPCR. We, therefore, tested 10 informative patients, at baseline and during clinical follow up, with both techniques. Overall, there was good concordance (r2=0.64) between the two methods (P<0.0001) in the 23 samples (18 BM, 5 PB) (Figure 3).
MYD88L265P digital droplet polymerase chain reaction on circulating tumor DNA
In order to investigate the feasibility and the sensitivity advantages of ddPCR-based MYD88L265P mutation detec- tion on plasma ctDNA vs. PB gDNA, paired samples from 60 patients were analyzed. Interestingly, a higher median MYD88L265P mutated / WT ratio was detected in plasma ctDNA (1.4x10-2) than in PB (1.03 x10-3) (P<0.001) (Online Supplementary Figure S5), while no statistically significant difference was observed between ctDNA and BM samples from 32 patients (1.92x10-2 vs. 1.4x10-2; P=0.2). Figure 4 shows the matches among BM, plasma and PB MYD88L265P
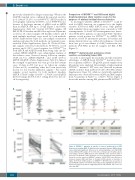
AB
C
Figure 1. MYD88L265P mutation at baseline is lower in peripheral blood (PB) than in bone marrow (BM). (A) Waldenström macroglobulinemia (WM) patients show statistically significant differences in mutated/wild-type ratio (MUT/WT) between BM and PB samples (P<0.0001). The control group of healthy subjects and multiple myeloma (MM) patients show MUT/WT ratios below the limit of detection. The dashed red line shows the cut-off for mutational status. Symbols below the red line represent MYD88WT samples. (B) MUT/WT ratio differences between relapsed (RE) and Naïve to Treatment (NT) patients, at baseline, is higher in PB than in BM samples. (C) MYD88L265P MUT/WT ratio at baseline in pared BM/PB samples from 55 NT and 19 RE patients, highlight the differences between biological specimens suggesting that for RE patients, PB is less reli- able than BM.
haematologica | 2018; 103(6)