Page 79 - Haematologica Vol. 107 - September 2022
P. 79
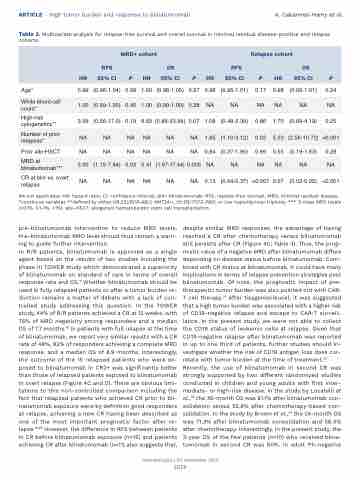
ARTICLE - High tumor burden and response to blinatumomab A. Cabannes-Hamy et al. Table 3. Multivariate analysis for relapse-free survival and overall survival in minimal residual disease-positive and relapse
cohorts.
MRD+ cohort
Relapse cohort
RFS
OS
RFS
OS
HR
95% CI
P
HR
95% CI
P
HR
95% CI
P
HR
95% CI
P
Age*
0.99
(0.96-1.04)
0.99
1.00
(0.96-1.05)
0.97
0.98
(0.95-1.01)
0.17
0.98
(0.95-1.01)
0.24
White blood cell count*
1.00
(0.99-1.00)
0.40
1.00
(0.99-1.00)
0.28
NA
NA
NA
NA
NA
NA
High-risk cytogenetics**
3.09
(0.56-17.0)
0.19
6.83
(0.86-53.99)
0.07
1.08
(0.48-2.39)
0.86
1.70
(0.69-4.19)
0.25
Number of prior relapses*
NA
NA
NA
NA
NA
NA
1.85
(1.10-3.12)
0.02
5.23
(2.56-10.72)
<0.001
Prior allo-HSCT
NA
NA
NA
NA
NA
NA
0.84
(0.37-1.95)
0.69
0.55
(0.19-1.63)
0.28
MRD at blinatumomab***
3.00
(1.15-7.84)
0.03
5.41
(1.67-17.44)
0.005
NA
NA
NA
NA
NA
NA
CR at blin vs. overt relapse
NA
NA
NA
NA
NA
NA
0.13
(0.04-0.37)
<0.001
0.07
(0.02-0.25)
<0.001
NA:not applicable; HR: hazard ratio; CI: confidence interval; blin: blinatumomab; RFS: replase-free survival; MRD: minimal residual disease. *continous variables **defined by either t(9;22)/BCR-ABL1, KMT2A-r, t(1;19)/TCF3-PBX1, or low hyploidy/near triploidy. *** 3-class MRD levels (<0.1%, 0.1-1%, >1%). allo-HSCT: allogeneic hematopoietic stem cell transplantation.
pre-blinatumomab intervention to reduce MRD levels. Pre-blinatumomab MRD level should thus remain a warn- ing to guide further intervention.
In R/R patients, blinatumomab is approved as a single agent based on the results of two studies including the phase III TOWER study which demonstrated a superiority of blinatumomab on standard of care in terms of overall response rate and OS.9 Whether blinatumomab should be used in fully relapsed patients or after a tumor burden re- duction remains a matter of debate with a lack of con- trolled study addressing this question. In the TOWER study, 44% of R/R patients achieved a CR at 12 weeks, with 76% of MRD negativity among responders and a median OS of 7.7 months.18 In patients with full relapse at the time of blinatumomab, we report very similar results with a CR rate of 48%, 82% of responders achieving a complete MRD response, and a median OS of 8.9 months. Interestingly, the outcome of the 15 relapsed patients who were ex- posed to blinatumomab in CR2+ was significantly better than those of relapsed patients exposed to blinatumomab in overt relapse (Figure 4C and D). There are obvious limi- tations to this non-controlled comparison including the fact that relapsed patients who achieved CR prior to bli- natumomab exposure were by definition good responders at relapse, achieving a new CR having been described as one of the most important prognostic factor after re- lapse.19,20 However, the difference in RFS between patients in CR before blinatumomab exposure (n=15) and patients achieving CR after blinatumomab (n=11) also suggests that,
despite similar MRD responses, the advantage of having reached a CR after chemotherapy versus blinatumomab still persists after CR (Figure 4C; Table 3). Thus, the prog- nostic value of a negative MRD after blinatumomab differs depending on disease status before blinatumomab. Com- bined with CR status at blinatumomab, it could have many implications in terms of relapse prevention strategies post blinatumomab. Of note, the prognostic impact of pre- therapeutic tumor burden was also pointed out with CAR- T cell therapy.21 After tisagenlecleucel, it was suggested that a high tumor burden was associated with a higher risk of CD19-negative relapse and escape to CAR-T surveil- lance. In the present study, we were not able to collect the CD19 status of leukemic cells at relapse. Given that CD19-negative relapse after blinatumomab was reported in up to one third of patients, further studies should in- vestigate whether the risk of CD19 antigen loss does cor- relate with tumor burden at the time of treatment.22 Recently, the use of blinatumomab in second CR was strongly supported by two different randomized studies conducted in children and young adults with first inter- mediate- or high-risk disease. In the study by Locatelli et al.,23 the 36-month OS was 81.1% after blinatumomab con- solidation versus 55.8% after chemotherapy-based con- solidation. In the study by Brown et al.,24 the 24-month OS was 71.3% after blinatumomab consolidation and 58.4% after chemotherapy. Interestingly, in the present study, the 3-year OS of the few patients (n=10) who received blina- tumomab in second CR was 80%. In adult Ph-negative
Haematologica | 107 September 2022
2078