Page 70 - Haematologica Vol. 107 - September 2022
P. 70
ARTICLE - Outcome of r/r B-ALL with extramedullary disease S. Kayser et al.
Veno-occlusive disease and sinusoidal obstruction syndrome
Up to four INO cycles were administered in patients as a bridge to transplantation (≤2 cycles, n=9; 3-4 cycles, n=3). Overall, VOD/SOS occurred in three (10%) patients, includ- ing one (8%) of 12 patients after transplantation. The first patient experienced VOD on the first day of the third INO cycle prior to allogeneic HSCT, but continued to trans- plantation after resolution and is in ongoing CR 24 months after transplant. The second patient developed VOD after three INO cycles and therefore stopped INO treatment. This patient did not proceed to allogeneic HSCT and is in CR 30.7 months after the start of INO treatment. The third patient received two cycles of INO prior to haplo-identical allogeneic HSCT with a conditioning regimen consisting of treosulfan/fludarabine/thiotepa. This patient developed VOD after transplantation and died 13.1 months after the transplant due to multi-organ failure and VOD.
Discussion
EMD is reported to occur in 20% of patients with ALL, being more common in patients with a T-cell phenotype, as well as in patients presenting with lymphoblastic lym- phoma, without bone marrow involvement.27 EMD may in- volve different sites, as observed in our series.28,29 The role of INO as treatment for patients with r/r ALL and EMD has largely not been studied. The randomized phase III INO- VATE trial included only seven r/r ALL patients with EMD given INO treatment as well as five patients treated with standard-of-care chemotherapy.30 Among patients with baseline EMD, five of seven (71%) in the INO arm and two of five (40%) in the standard care arm achieved CR/CRi,
which included resolution of EMD.30 Consistent with pre- vious reports on the effectiveness of INO in patients with EMD, we observed a high CR rate of 55% after INO treat- ment in patients with r/r ALL and EMD.30-32 There was no difference based on the presence or absence of concur- rent bone marrow disease. Additionally, the median OS of 12.8 months in our cohort of heavily pretreated patients compares favorably to that of the less heavily pre-treated patients enrolled in the INO-VATE trial (7.7 months), al- though the number of patients with EMD in the aforemen- tioned trial was very low limiting the comparison.31 The high response rate of ALL with EMD treated with INO may be an advantage of INO treatment since the presence or history of EMD may predict poor responses to other ther- apies, specifically blinatumomab.19 In a retrospective co- hort study of 65 patients with r/r ALL, a high leukemia burden, defined as bone marrow blast cells >50% (odds ratio =0.24; P=0.02) as well as presence of EMD (odds ratio =0.19; P=0.05) or history of EMD (odds ratio =0.23; P=0.005) were associated with lower response to blina- tumomab.19 It remains unknown whether increasing the dose of blinatumomab for ALL would be able to overcome this resistance (and be tolerable), since a higher dose has been studied for non-Hodgkin lymphomas and produced reasonable results.33 In contrast to blinatumomab,34 only a few cases of CD22 antigen loss have been described so far.35,36 In our cohort, we did not observe any CD22 antigen loss. In the INO-VATE trial, the inclusion of a small group of patients who were CD22-negative or had low CD22 ex- pression was reported. Interestingly, three of five of these patients showed a response to INO treatment.15 Fur- thermore, response in a CD22-negative patient was also described in a case report.37 Thus, INO might be active in CD22-negative patients and/or those with very dim CD22
Haematologica | 107 September 2022
2069
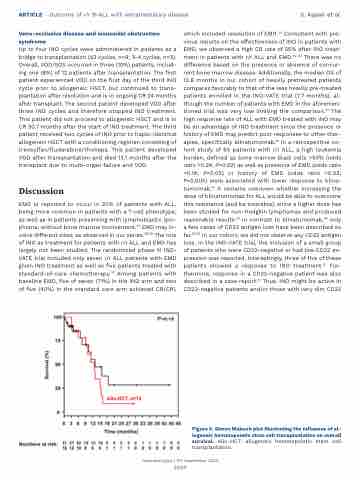
Figure 5. Simon Makuch plot illustrating the influence of al- logeneic hematopoietic stem cell transplantation on overall survival. Allo-HCT: allogeneic hematopoietic stem cell transplantation.