Page 40 - Haematologica Vol. 107 - September 2022
P. 40
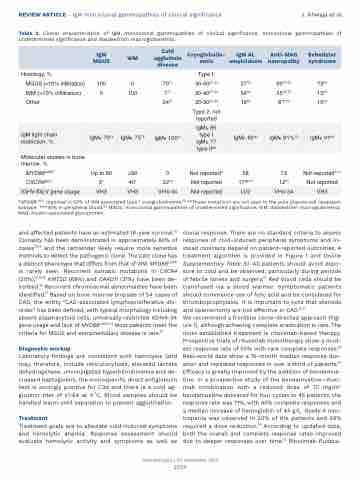
REVIEW ARTICLE - IgM monoclonal gammopathies of clinical significance J. Khwaja et al. Table 2. Clonal characteristics of IgM monoclonal gammopathies of clinical significance, monoclonal gammopathies of
undetermined significance and Waldeström macroglobinemia.
IgM MGUS
WM
Cold agglutinin disease
Cryoglobulin- emia
IgM AL amyloidosis
Anti-MAG neuropathy
Schnitzler syndrome
Histology, %
Type I:
MGUS (<10% infiltration)
100
0
7071
30-4027,30
2740
6072,73
7363
WM (>10% infiltration)
0
100
771
30-4027,30
5440
3572,73
1363
Other
2471
20-3027,30
1940
872,73
1563
Type 2: not reported
IgM light chain restriction, %
IgMk 7074
IgMk 7575
IgMv 10011
IgMk 85 type I IgMk 77 type II26
IgMλ 6040
IgMk 81%72
IgMk 9163
Molecular studies in bone marrow, %
MYD88L265P
Up to 80
>90
0
Not reported*
58
73
Not reported***
CXCR4MUT
57
407
2215
Not reported
1738**
1272
Not reported
IGHV/IGLV gene usage
VH3
VH3
VH4-34
Not reported
LV2
VH4-34
VH3
*MYD88L265P reported in 92% of WM associated type I cryoglobulinemia.76 **These mutations are not seen in the pure plasma cell neoplasm subtype. ***30% in peripheral blood.66 MGUS: monoclonal gammopathies of undetermined significance; WM: Waldeström macroglobinemia; MAG: myelin-associated glycoprotein.
and affected patients have an estimated 16-year survival.10 Clonality has been demonstrated in approximately 80% of cases10,14 and the remainder likely require more sensitive methods to detect the pathogenic clone. The CAD clone has a distinct phenotype that differs from that of WM. MYD88L265P is rarely seen. Recurrent somatic mutations in CXCR4 (20%),10,14,15 KMT2D (69%) and CARD11 (31%) have been de- scribed.16 Recurrent chromosomal abnormalities have been identified.17 Based on bone marrow biopsies of 54 cases of CAD, the entity "CAD-associated lymphoproliferative dis- order" has been defined, with typical morphology including absent plasmacytoid cells, universally restricted IGHV4-34 gene usage and lack of MYD88L265P.14 Most patients meet the criteria for MGUS and extramedullary disease is rare.10
Diagnostic workup
Laboratory findings are consistent with hemolysis (and may, therefore, include reticulocytosis, elevated lactate dehydrogenase, unconjugated hyperbilirubinemia and de- creased haptoglobin), the monospecific direct antiglobulin test is strongly positive for C3d and there is a cold ag- glutinin titer of ≥1:64 at 4˚C. Blood samples should be handled warm until separation to prevent agglutination.
Treatment
Treatment goals are to alleviate cold-induced symptoms and hemolytic anemia. Response assessment should evaluate hemolytic activity and symptoms as well as
clonal response. There are no standard criteria to assess response of cold-induced peripheral symptoms and in- stead clinicians depend on patient-reported outcomes. A treatment algorithm is provided in Figure 1 and Online Supplementary Table S1. All patients should avoid expo- sure to cold and be observed, particularly during periods of febrile illness and surgery.10 Red blood cells should be transfused via a blood warmer. Symptomatic patients should commence use of folic acid and be considered for thromboprophylaxis. It is important to note that steroids and splenectomy are not effective in CAD.9,12
We recommend a frontline clone-directed approach (Fig- ure 1), although achieving complete eradication is rare. The most established treatment is rituximab-based therapy. Prospective trials of rituximab monotherapy show a mod- est response rate of 50% with rare complete responses.18 Real-world data show a 15-month median response dur- ation and repeated responses in over a third of patients.10 Efficacy is greatly improved by the addition of bendamus- tine. In a prospective study of the bendamustine-rituxi- mab combination with a reduced dose of 70 mg/m2 bendamustine delivered for four cycles to 45 patients, the response rate was 71%, with 40% complete responses and a median increase of hemoglobin of 44 g/L. Grade 4 neu- tropenia was observed in 20% of the patients and 29% required a dose reduction.19 According to updated data, both the overall and complete response rates improved due to deeper responses over time.10 Rituximab-fludara-
Haematologica | 107 September 2022
2039