Page 282 - Haematologica Vol. 107 - September 2022
P. 282
CASE REPORT
ABC
DEF
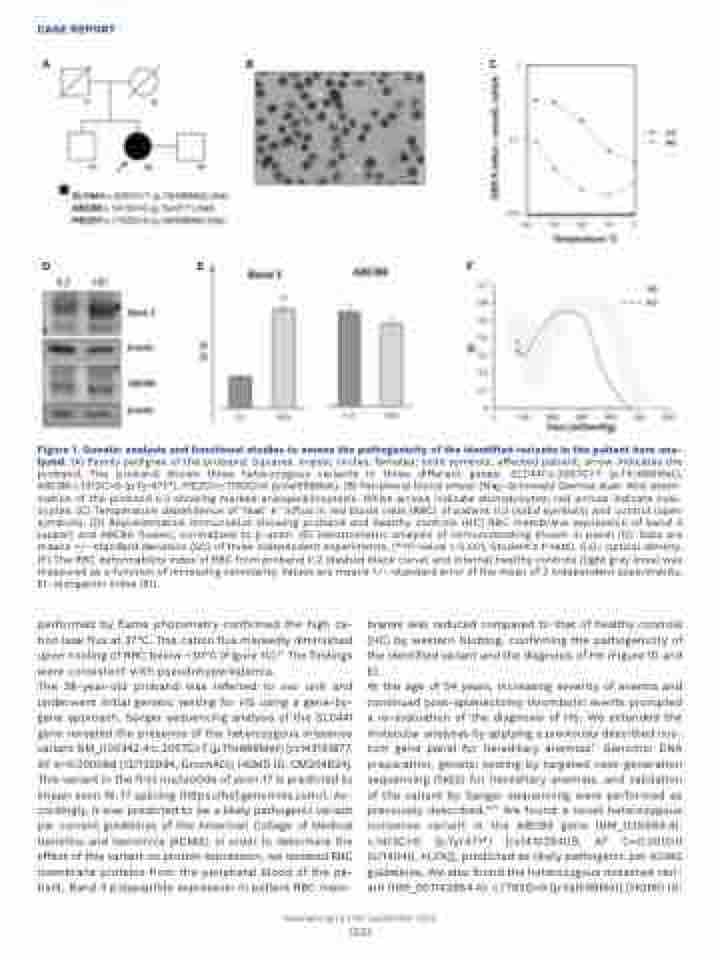
Figure 1. Genetic analysis and functional studies to assess the pathogenicity of the identified variants in the patient here ana- lyzed. (A) Family pedigree of the proband. Squares, males; circles, females; solid symbols, affected patient; arrow indicates the proband. The proband shows three heterozygous variants in three different genes: SLC4A1:c.2057C>T (p.Thr686Met), ABCB6:c.1413C>G (p.Tyr471*), PIEZO1:c.1792G>A (p.Val598Met). (B) Peripheral blood smear (May-Grünwald Giemsa stain 40x) exam- ination of the proband II.2 showing marked anisopoikilocytosis. White arrows indicate stomatocytes; red arrows indicate oval- ocytes. (C) Temperature dependence of ‘leak’ K+ influx in red blood cells (RBC) of patient II.2 (solid symbols) and control (open symbols). (D) Representative immunoblot showing proband and healthy controls (HC) RBC membrane expression of band 3 (upper) and ABCB6 (lower), normalized to b-actin. (E) Densitometric analysis of immunoblotting shown in panel (D). Data are means +/- standard deviation (SD) of three independent experiments. (**P-value < 0.001, Student’s t-test). O.D.: optical density. (F) The RBC deformability index of RBC from proband II.2 (dashed black curve) and internal healthy controls (light grey lines) was measured as a function of increasing osmolarity. Values are means +/– standard error of the mean of 2 independent experiments. EI: elongation index (EI).
performed by flame photometry confirmed the high ca- tion leak flux at 37°C. This cation flux markedly diminished upon cooling of RBC below ~30°C (Figure 1C).10 The findings were consistent with pseudohyperkalemia.
The 38-year-old proband was referred to our unit and underwent initial genetic testing for HS using a gene-by- gene approach. Sanger sequencing analysis of the SLC4A1 gene revealed the presence of the heterozygous missense variant NM_000342.4:c.2057C>T (p.Thr686Met) [rs143131877, AF A=0.000088 (12/135994, GnomAD); HGMD ID: CM204624]. This variant in the first nucleotide of exon 17 is predicted to impair exon 16-17 splicing (https://hsf.genomnis.com/). Ac- cordingly, it was predicted to be a likely pathogenic variant per current guidelines of the American College of Medical Genetics and Genomics (ACMG). In order to determine the effect of this variant on protein expression, we isolated RBC membrane proteins from the peripheral blood of the pa- tient. Band 3 polypeptide expression in patient RBC mem-
branes was reduced compared to that of healthy controls (HC) by western blotting, confirming the pathogenicity of the identified variant and the diagnosis of HS (Figure 1D and E).
At the age of 54 years, increasing severity of anemia and continued post-splenectomy thrombotic events prompted a re-evaluation of the diagnosis of HS. We extended the molecular analyses by applying a previously described cus- tom gene panel for hereditary anemias.11 Genomic DNA preparation, genetic testing by targeted next-generation sequencing (NGS) for hereditary anemias, and validation of the variant by Sanger sequencing were performed as previously described.11,12 We found a novel heterozygous nonsense variant in the ABCB6 gene (NM_005689.4): c.1413C>G (p.Tyr471*) [rs141029409, AF C=0.00000 (0/14046, ALFA)], predicted as likely pathogenic per ACMG guidelines. We also found the heterozygous missense vari- ant (NM_001142864.4): c.1792G>A (p.Val598Met) [HGMD ID:
Haematologica | 107 September 2022
2281