Page 258 - Haematologica Vol. 107 - September 2022
P. 258
LETTER TO THE EDITOR
low-up time off lymphoma therapy of 42 months; two pa- tients (#1 and #6) were included in the NIVOALCL trial (NCT03703050) and received nivolumab while still in com- plete remission; and one patient (#5) definitively stopped the next-generation ALK inhibitor and received no further therapy, with more than 10 months off treatment at the date of last follow-up considered for the study.
Overall, nine out of ten patients were alive in complete remission at the date of last visit. The median duration of the follow-up from the initiation of ALK inhibitor treat- ment in these nine patients was 24.2 months (range, 11.3-
48.1).
Of note, next-generation ALK inhibitors could be helpful in critically ill patients as clinical improvements occurred very fast; for example, patient #3 was in a coma at treat- ment initiation and regained normal consciousness. Re- sponse on imaging was also impressive for the patients with intracranial masses (Online Supplementary Figure S1). Regarding tolerance of next-generation ALK inhibitors, we reported notable adverse events of grade 3 or higher in eight patients, including weight gain in three patients, neuropsychological manifestations in three patients and
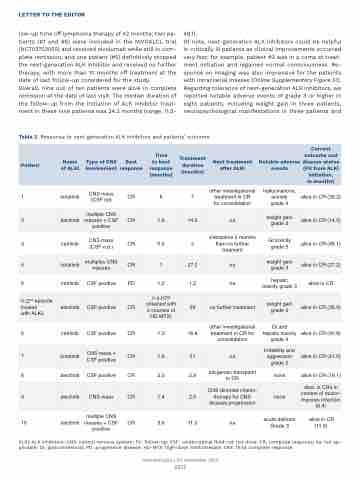
Table 2. Response to next-generation ALK inhibitors and patients’ outcome.
Patient
Name of ALKi
Type of CNS involvement
Best response
Time to best response (months)
Treatment duration (months)
Next treatment after ALKi
Notable adverse events
Current outcome and disease status (FU from ALKi initiation,
in months)
1
lorlatinib
CNS mass (CSF nd)
CR
6
7
other investigational treatment in CR for consolidation
hallucinations, anxiety grade 3
alive in CR (30.2)
2
alectinib
multiple CNS masses + CSF positive
CR
1.6
14.5
na
weight gain grade 3
alive in CR (14.5)
3
ceritinib
CNS mass (CSF n.d.)
CR
0.5
3
vinblastine 3 months then no further treament
GI toxicity grade 3
alive in CR (48.1)
4
lorlatinib
multiples CNS masses
CR
1
27.2
na
weight gain grade 3
alive in CR (27.2)
5
ceritinib
CSF positive
PD
1.2
1.2
na
hepatic toxicity grade 3
alive in CR
5 (2nd episode treated
with ALKi)
alectinib
CSF positive
CR
n.a.(CR obtained with 2 courses of HD MTX)
26
no further treatment
weight gain grade 3
alive in CR (36.5)
6
ceritinib
CSF positive
CR
1.3
16.9
other investigational treatment in CR for consolidation
GI and hepatic toxicity grade 3
alive in CR (40.8)
7
lorlatinib
CNS mass + CSF positive
CR
1.6
21
na
irritability and aggression grade 2
alive in CR (21.0)
8
alectinib
CSF positive
CR
2.5
2.9
allogeneic transplant in CR
none
alive in CR (19.1)
9
alectinib
CNS mass
CR
1.4
2.5
CNS-directed chemo- therapy for CNS disease progression
none
died, in CR3 in context of mucor- mycosis infection (9.4)
10
alectinib
multiple CNS masses + CSF positive
CR
3.6
11.3
na
acute delirium Grade 3
alive in CR (11.3)
ALKi: ALK inhibitors; CNS: central nervous system; FU: follow-up; CSF: cerebrospinal fluid; nd: not done; CR: complete response; na: not ap- plicable; GI: gastrointestinal; PD: progressive disease; HD-MTX: high-dose methotrexate; CR3: third complete response.
Haematologica | 107 September 2022
2257