Page 219 - Haematologica May 2022
P. 219
Case Reports
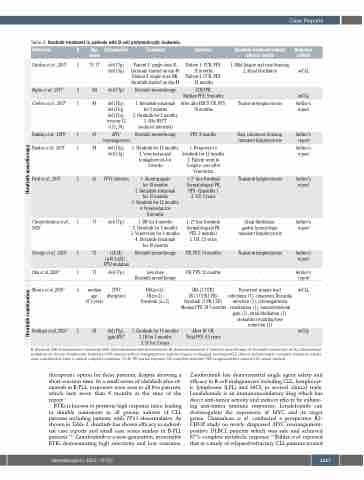
Table 2. Ibrutinib treatment in patients with B-cell prolymphocytic leukemia.
Reference N
Age, years
73, 77
NA 48
67 84
66
77
73
71
median age: 67.3 years
68
Gordon et al., 20172
Algrin et al., 20173 Coelho et al., 20174
Damlaj et al., 20185 Bindra et al., 20196
Patil et al., 20197
Christoforidou et al., 20208
George et al., 20209
Oka et al.,202010 Moore et al.,202011
Siddiqui et al.,202112
2
4 1
1 1
1
1
1
1 6
1
Cytogenetics
del(17p) del(13q)
del(17p)
del(17p), del(11q), del(13q),
trisomy 12, t(11; 14)
MYC
rearrangement
del(17p), del(13q)
TP53 deletion
del(17p)
t(4;14) (p16.3;q32) , TP53 mutation
Treatment
Patient 1: single dose R, Ibrutinib started on day 40 Patient 2: single dose BR, Ibrutinib started on day 44
Ibrutinib monotherapy
1. Idelalisib-rituximab for 5 months
2. Ibrutinib for 2 months 3. Allo-HSCT (reduced intensity)
Ibrutinib monotherapy
1. Ibrutinib for 12 months 2. Venetoclax and leukapheresis for
5 weeks
1. Alemtuzumab
for 18 months
2. Idelalisib-rituximab for 12 months
3. Ibrutinib for 12 months 4. Venetoclax for
8 months
1. BR for 4 months
2. Ibrutinib for 5 months 3. Venetoclax for 6 months 4. Idelalisib-rituximab for 10 months
Ibrutinib monotherapy
Low dose Ibrutinib monotherapy
IRA(n=2); IR(n=2); Ibrutinib (n=2)
1. Ibrutinib for 10 months 2. IR for 3 months
3. IV for 3 years
Outcome
Patient 1: CCR, PFS: 15 months Patient 2: CCR, PFS: 12 months
3CR/1PR, Median PFS: 9 months
After allo-HSCT: CR, PFS: 10 months
PFS: 8 months
1. Response to ibrutinib for 12 months 2. Patient went to hospice care after Venetoclax
1. 3rd line Ibrutinib (hematological PR, PFS: 12months ) 2. OS: 5 years
1. 2nd line Ibrutinib (hematological PR, PFS: 5 months) 2. OS: 2.5 years
PR, PFS: 15 months
CR, PFS: 12 months
IRA (2 CCR)
IR (1 CCR,1 PR) Ibrutinib (1 PR,1 SD) Median PFS: 34.7 months
After IV: CR; Total PFS: 4.5 years
Ibrutinib treatment-related adverse events
1. Mild fatigue and easy bruising, 2. Atrial fibrillation
-
Transient lymphocytosis
Easy cutaneous bruising, transient lymphocytosis
-
Transient lymphocytosis
Atrial fibrillation, gastric hemorrhage, transient lymphocytosis
Transient lymphocytosis
-
Recurrent urinary tract infections (1), cutaneous Nocardia infection (1), cytomegalovirus reactivation (1), musculoskeletal pain (3), atrial fibrillation (1), stomatitis requiring dose reduction (1)
-
Response criteria
iwCLL
iwCLL
Author’s report
Author’s report
Author’s report
Author’s report
Author’s report
Author’s report
Author’s report
iwCLL
iwCLL
del(17p)
TP53
disruption
del(17p), gain MYC
R: rituximab; BR: bendamustine + rituximab; IRA: ibrutinib+rituximab+alemtuzumab; IR: ibrutinib+rituximab; I: ibrutinib monotherapy; IV: ibrutinib+venetoclax; iwCLL: international workshop on chronic lymphocytic leukemia; CCR: patients without restaging bone marrow biopsies or imaging, meeting iwCLL clinical and laboratory complete remission criteria were considered to have a clinical complete remission (CCR); PR: partial response; CR: complete response; PFS: progression-free survival; OS: overall survival.
therapeutic option for these patients, despite showing a short reaction time. In a small series of idelalisib plus rit- uximab in B-PLL, responses were seen in all five patients, which lasts more than 6 months at the time of the report.1
BTKi is known to promote high response rates, leading to durable remissions in all genetic subsets of CLL patients including patients with TP53 abnormalities. As shown in Table 2, ibrutinib has shown efficacy in individ- ual case reports and small case series studies in B-PLL patients.2-12 Zanubrutinib is a new-generation, irreversible BTKi demonstrating high selectivity and low toxicities.
Zanubrutinib has demonstrated single agent safety and efficacy in B-cell malignancies including CLL, lymphocyt- ic lymphoma (LPL) and MCL in several clinical trials. Lenalidomide is an immunomodulatory drug which has direct anti-tumor activity and indirect effects by enhanc- ing anti-tumor immune responses. Lenalidomide can downregulate the expression of MYC and its target genes. Chamuleau et al. conducted a prospective R2- CHOP study on newly diagnosed MYC rearrangement- positive DLBCL patients which was safe and achieved 67% complete metabolic response.13 Bühler et al. reported that in a study of relapsed/refractory CLL patients treated
haematologica | 2022; 107(5)
1227
Ibrutinib combination Ibrutinib monotherapy