Page 208 - Haematologica May 2022
P. 208
Letters to the Editor
recent CIBMTR analysis that patients with a PR prior to autoHCT had a 5-year PFS of 41%.12 As such, patients with chemosensitive disease, particularly those who attain a CR, should not be denied the opportunity for curative intent treatment with autoHCT solely due to the number of prior lines of therapy.
We acknowledge a number of limitations with this analysis including its retrospective design as well as that these data pertain only to patients who respond to sal- vage therapy, and many patients do not.13 Registry data show that the number of patients who undergo autoHCT in the 3L+ setting is less than those who do so
after two lines of therapy.10,14 Of the patients in the CORAL study who did not respond to second-line ther- apy, only 39% responded to 3L therapy and 28% of patients ultimately proceeded to autoHCT.15 Furthermore, many novel therapies for DLBCL have been approved recently including multiple targeted treatments as well as three separate CAR-T products.3-5 CAR-T therapy has profoundly impacted the care of DLBCL given its ability to induce durable remissions even in the setting of chemorefractory disease. Although only approved in the third line setting at present, ran- domized trials of CAR-T compared to salvage chemoim-
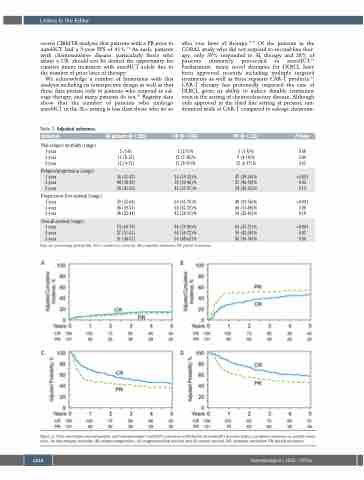
Table 2. Adjusted outcomes. Outcomes
All patients (N = 285)
CR (N = 154)
5 (2-9)% 12 (7-18)% 13 (8-19)%
26 (19-33)% 38 (30-46)% 45 (37-53)%
69 (61-76)% 50 (42-59)% 42 (34-51)%
84 (79-90)% 64 (56-72)% 56 (48-65)%
P-Value 5 (1-8)% 0.68
9 (4-14)% 0.40 12 (6-17)% 0.65
Non-relapse mortality (range)
1-year 5 (3-8) 3-year 11 (8-15) 5-year 12 (9-17)
Relapse/progression (range)
1-year 36 (31-42) 3-year 44 (38-50) 5-year 50 (43-56)
Progression-free survival (range)
1-year 59 (53-64) 3-year 45 (39-51) 5-year 38 (32-44)
Overall survival (range)
1-year 74 (69-79) 3-year 57 (51-63) 5-year 51 (44-57)
47 (39-56)%
51 (42-59)% 0.03 54 (45-63)% 0.14
PR (N = 131)
63 (55-71)%
50 (42-59)% 0.02 45 (36-54)% 0.06
<0.001
48 (39-56)%
40 (31-48)% 0.08 34 (26-43)% 0.18
<0.001
<0.001
Data are percentage probability (95% confidence interval). CR: complete remission; PR: partial remission.
AB
CD
Figure 1. Post- autologous hematopoietic cell transplantation (autoHCT) outcomes stratified by pre-autoHCT disease status (complete response vs. partial remis- sion). (A) Non-relapse mortality, (B) relapse/progression, (C) progression-free survival and (D) overall survival. CR: complete remission; PR: partial remission.
1216
haematologica | 2022; 107(5)