Page 121 - 2022_03-Haematologica-web
P. 121
O-linked glycans protect VWF
tures. Importantly, glycoprotein aging in plasma is asso- ciated with progressive loss of capping sialic acid, and thus increased exposure of these sub-terminal Gal residues.38
There are previous reports of significantly increased binding of RCA-I lectin to plasma VWF in patients with VWD.10,33,40,42 This lectin binds preferentially to Gal or GalNAc sugars which are typically present as sub-termi- nal residues on the O- and N-glycans of pdVWF, but become exposed following loss of capping sialic acid. Increased RCA-I binding has also been correlated with enhanced VWF clearance in VWD patients.10,33,40 Our data suggest that the shortened half-life associated with increased Gal exposure (and hence RCA-I binding) in VWD patients is mediated in large part through enhanced MGL-mediated clearance. Importantly, van Schooten et al. previously reported significantly increased binding of peanut agglutinin (PNA) lectin to VWF in a cohort of VWD patients.40 This lectin preferentially binds to the T antigen structure which is exposed following loss of O- linked sialylation. The authors further showed that increased PNA-binding (T antigen exposure) was associ- ated with a significant increase in the VWFpp/VWF:Ag ratio, consistent with enhanced VWF clearance.40 In keep- ing with these results, we have demonstrated that α2-3
AB
linked sialylation on O-linked glycan structures plays a particular role in protecting VWF against MGL-mediated clearance. Consequently, our findings suggest that the enhanced clearance associated with T antigen exposure on VWF previously reported by van Schooten et al. is attributable to enhanced clearance via MGL.
Besides VWD, abnormal VWF glycosylation has also been reported in a number of other disease states.24,39-41 For example, reduced PNA-binding to VWF has been reported in patients with liver cirrhosis who have signif- icantly elevated plasma VWF:Ag levels. The biological mechanisms underlying reduced T antigen exposure on VWF in patients with cirrhosis have not been defined. Nonetheless, our findings build upon these previous observations and in particular suggest that the altered O- glycosylation associated with cirrhosis will cause increased plasma VWF levels as a result of decreased MGL-mediated clearance. Conversely, a number of dif- ferent pathogens including Streptococcus pneumoniae, Haemophilus influenzae and Pseudomonas aeruginosa express neuraminidase enzymes that can cause desialyla- tion of host glycoproteins.24,54 VWF desialylation associat- ed with pathological, enhanced clearance has been observed in mice infected with S. pneumoniae.24 Our data further suggest that increased MGL-mediated clearance
C
DE
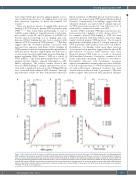
Figure 7. α2-3 linked sialic acid on platelet von Willebrand factor protects from enhanced circulatory clearance. (A-C) Platelet-derived (plt) von Willebrand factor (VWF) sialylation was assessed using lectin binding assays with Sambucus nigra (A), Maackia amuresis (B) and Ricinus communis (C). Plasma-derived (pd) VWF was used as a control. (D) Solid phase binding assay was used to assess the binding of plt-VWF to immobilized human MGL and again compared to human pd-VWF. (E) In vivo pharmacokinetic experiments were performed in VWF-/- mice to compare the clearance rates or plt-VWF compared to pd-VWF. At each time point, residual cir- culating VWF:antigen (VWF:Ag) concentration was determined by enzyme-linked immunosorbent assay. All results are plotted as percentage residual VWF:Ag levels relative to the amount injected. Data are represented as mean ± standard error of mean (SEM). In some cases, the SEM cannot be seen because of its small size. *P<0.05, **P<0.01, ns: not significant.
haematologica | 2022; 107(3)
677