Page 332 - 2022_01-Haematologica-web
P. 332
Letters to the Editor
D
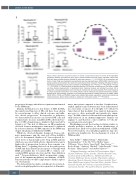
Figure 2. Mass cytometry: supervised analysis (A and B), unsupervised analysis (C and D). (A) In responders (comparing baseline [pre-induction] time point to pooled maintenance time points), activated natural killer (NK) cells were enriched whereas inhibited NK cells were reduced. (****P<0.0001). (B) In responders, reg- ulatory T cells (Treg) were depleted by pomalidomide low-dose dexamethasone (POM-LoDEX) induction (com- paring baseline [pre-induction] time point with maintenance C1D1 timepoint). Following withdrawal of DEX in maintenance, some Treg recovery seen in patients on the POM only arm (*P<0.05, ****P<0.0001) (*P<0.05, **** P<0.0001). (C) Cluster analysis at baseline identified 131 immune cell populations. Plot shows a single cell force directed representation of peripheral blood. Individual clusters are indicated by colours. (D) In responders, neutrophil populations were enriched at maintenance time points (pooled). Plots show frequency of unique neutrophil clusters #1-5 out of total patients’ cells for induction and pooled maintenance samples. Example brick plot phenotype (indicative of all neutrophil populations) showing expression of CD66b, CD24, CD16, CD11c, CD11b and CD45RO. Large bricks indicate high relative expression, small bricks indicate low relative expression. Absent bricks indicate no expression of the given marker. (****P<0.0001)
progression therapy, which favored patients randomized to the POM arm.
Immune dysfunction is a key feature of MM. In MM, the number and function of NK cells have been shown by several groups to affect clinical outcome, and influ- ence disease progression.9 In responders to induction, we demonstrated an increase in activated NK cells and commensurate decrease in inhibited NK cells from base- line to C1D1 of maintenance, similar to that reported by Sehgal et al.3 The lack of difference in NK populations observed between maintenance arms may be explained by a shorter duration of POM exposure in the POM arm despite the planned withdrawal of DEX.
Whilst we observed dynamic changes in Treg accord- ing to maintenance arm, the exact role of Treg in MM is yet to be determined. Muthu et al.10 have reported ele- vated levels of functionally active Treg in MM patients which are associated with adverse clinical features and a higher risk of progression, however there remains con- flicting data11,12 regarding their role in the pathogenesis of MM and their alterations in response to therapy with IMiD, potentially due to location (PB vs. tumor), con- comitant DEX, patient selection and the Treg definition used.13 Treg modulation is likely an important compo- nent of the immunomodulatory mechanisms of IMiD. Functional studies would be important to further explore our observations.
We demonstrated a relative enrichment of several acti- vated neutrophil populations in responders at all mainte-
nance time points compared to baseline. Peripheral neu- trophil expansion and activation has been demonstrated in a vast array of cancers. It is thought to be driven by tumor factors that modulate bone marrow hemopoietic processes to drive neutrophil and granulocyte expan- sion.14 In MM, it has been shown that neutrophils poten- tially function in an immunosuppressive manner via arginase-1, and therefore could contribute to both dis- ease progression and sepsis.15
Our findings provide the baseline for future studies to identify predictive markers to allow identification of patients more likely to benefit from withdrawal of DEX. Novel observations of neutrophil populations may also provide new insights into the mechanisms of action of POM in MM.
Anna Kalff,1,2,3 Tiffany Khong,1,2 Malarmathy Ramachandran,1,2 P Joy Ho,4 Peter Mollee,5 James D’Rozario,6 Kerry Taylor,7 Jane Estell,8 Sam Norton,9 10 Roslyn Kemp,10 Andrew J. Mitchell,11 John Reynolds,12 Nola Kennedy,1 Hang Quach13 and Andrew Spencer1,2,3
1Malignant Hematology and Stem Cell Transplantation, Alfred Hospital, Melbourne, Victoria, Australia; 2Myeloma Research Group, Australian Center for Blood Diseases, Alfred Hospital Monash University, Melbourne, Victoria, Australia; 3Department of Clinical Hematology, Monash University, Clayton, Victoria, Australia; 4Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; 5Princess Alexandra Hospital and University of Queensland, Brisbane, Queensland, Australia; 6The Canberra Hospital, Canberra, New
324
haematologica | 2022; 107(1)