Page 172 - 2022_01-Haematologica-web
P. 172
T. Wang et al.
AB
CDF
E
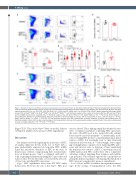
Figure 7. Deletion of Nupr1 in adulthood promotes hematopoietic stem cell engraftment. (A) Cell cycle analysis of Nupr1fl/flMx1-cre hematopoietic stem cells (HSC) under homeostasis. Representative plots of cell cycle from representative wild-type (WT) and Nupr1fl/flMx1-cre mice (8 weeks old). WT littermates (8 weeks old) were used as controls. HSC (Lin– (i.e., CD2, CD3– CD4– CD8– B220– Gr1– CD11b– Ter119–) CD48– Sca1+ c-kit+ CD150+ CD34– CD135–) were analyzed by DNA content (DAPI) versus Ki-67. G0 (Ki-67lowDAPI2N), G1 (Ki-67highDAPI2N), G2-S-M (Ki-67highDAPI>2N-4N). (B) Statistical analysis of the number of long-term HSC from WT and Nupr1fl/flMx1- cre mice. BMNC: bone marrow nucleated cells. (C) Statistical analysis of the cell cycle of HSC. Ctr: n=4, Nupr1fl/flMx1-cre, n=5. **P<0.01. (D) Kinetic analysis of donor chimerism (CD45.2+) in peripheral blood. Data were analyzed by two-way analysis of variance and are represented as mean ± SD (Ctr group: n = 5 mice, Nupr1fl/flMx1-cre group: n =7 mice). ***P<0.001. (E) Flow cytometry analysis of the HSC compartment in primary recipients 4 months after transplantation. (F) Statistical analysis of donor HSC number and percentage in the transplantation chimeras. Data were analyzed using an unpaired Student t-test and are represented as mean ± standard deviation, n=5. **P<0.01, ***P<0.001.
(Figure 7E, F). Thus, in the Nupr1fl/fl Mx1-cre model, deletion of Nupr1 in adulthood also promotes HSC engraftment.
Discussion
The intrinsic networks regulating the quiescence of HSC are largely unknown. In this study, loss of Nupr1 (p8), a gene preferentially expressed in long-term HSC, mildly tuned the quiescence threshold of HSC in the state of homeostasis, without compromising their essential func- tions in hematopoiesis. Nupr1 coordinated with p53 to form a signaling machinery regulating HSC quiescence and turnover rates. For the first time, we revealed the new role of Nupr1 in controlling HSC quiescence.
Nupr1-/- HSC replenished faster than WT HSC under homeostasis. However, the size of the Nupr1-/- HSC pool
was not altered. These findings imply that despite the exis- tence of intrinsic machinery controlling HSC quiescence, the scale of the HSC pool is also restricted by the extrinsic bone marrow microenvironment.48 Conventionally, mole- cules activating HSC produce a transient phenotypic prolif- eration of HSC but eventually lead to their functional exhaustion and even tumors.36-40 Interestingly, Nupr1 signal- ing seemingly plays a unique role in regulating HSC quies- cence and turnover rates, as deletion of Nupr1 maintained the hematopoietic features of HSC. Consistently, enforced CDK6 expression in HSC confers these cells a competitive advantage without impairing their stemness and multilin- eage potential.9 This evidence supports the concept that tar- geting the intrinsic machinery of balancing the threshold of HSC quiescence might safely promote engraftment.
Loss of Nupr1 in HSC resulted in an engraftment advan- tage. In the setting of transplantation stress, the HSC
164
haematologica | 2022; 107(1)