Page 18 - 2021_12-Haematologica-web
P. 18
ChAd3
7 Vena cava thrombosis (n=1)
5 NR 2 NR
NR NR NR
P. Gresele et al.
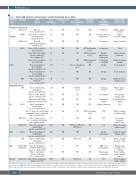
Table 1. Studies with adenovirus-vectored vaccines reporting hematologic adverse effects.
Adenoviral Pathogens vector
Chimpanzee Adenovirus
Influenza A
MERS ChAdOx1
SARS-CoV-2
Study (type of)
Phase Ia, dose-escalation (S1)
Phase I, dose-escalation, non-randomized, uncontrolled (64)
Phase I/II, single-blind, randomized, controlled (70)
N. of Thrombocytopenia participants (n)
15 NR 24 NR
1077 NR
Venous thromboembolism (n)
Coagulation disorders
NR NR
NR
Other hematologic complications
Leukopenia
Anemia, neutropenia, lymphopenia
Neutropenia
Other systemic adverse events
Fatigue, malaise, headache
Fatigue, headache, myalgia
Fatigue, headache
Human Adenovirus
Phase I, double-blind, randomized, placebo-controlled (66)
Phase I, double-blinded, placebo-controlled (S8)
Phase IIb, double-blind, randomized, controlled (S9)
Phase I, randomized, double-blind, placebo-controlled (S10)
Phase I, single-site, double-blind, randomized, placebo-controlled, dose-escalation (S11)
114 NR
36 NR 801 NR 120 NR
32 NR 108 4
58 NR
48 1 72 NR
Phlebitis (n=1)
NR NR NR
NR NR
DVT (n=1)
NR NR
NR
NR NR NR
aPTT prolongation (n=2)
NR
NR
NR NR
Leukopenia, anemia
Neutropenia
Neutropenia, anemia
Anemia, leukopenia NR
NR
NR
WBC count abnormalities
Neutropenia, eosinophilia
Fatigue, malaise, headache
Fever, malaise, myalgia, chills
Headache, malaise, myalgia
Fever
Malaise, myalgia, headache, chills
Fever, fatigue, headache, muscle pain
Malaise, myalgia, headache, chills
Myalgia, chills, headache, fever, vomiting Fever, headache, malaise, myalgia, nausea
Ad5
HIV
Ebola
Ad26
SARS-CoV-2 Ebola
Phase III, randomized, double-blind, placebo-controlled (71)
Phase II, randomized, double-blind, placebo-controlled (S13)
44325 423
NR DVT (n=6), PE (n=4), NR CSVT (n=1)
NR NR NR
NR
Anemia, neutropenia
Fatigue, headache, myalgia, nausea
Fatigue, headache, myalgia, chills
Ad35
SARS-Cov2
HIV
Plasmodium Falciparum
Phase I, single-centre, dose-escalation, double-blind, non-randomized (S12)
Phase I, double-blind, randomized, placebo-controlled (67)
Phase Ib, randomized, controlled, double-blind, dosage-escalation (S14) Phase I, randomized, placebo-controlled, dose-escalation (S15)
Ebola
Phase I, dose-escalation, 20 open-label (S2)
Phase I/IIa, double-blind, 120 placebo-controlled,
dose-finding (S3)
Phase I, dose-escalation, 60 open-label (S4)
Phase II, randomized, 3030 observer-blind,
placebo-controlled (S5)
Phase II, randomized, 600 observer-blind,
placebo-controlled (S6)
Phase I, open-label, 42 single-site,
dose-escalation (S7)
NR NR NR NR
1 NR
aPTT prolongation (15%)
aPTT prolongation (n=1)
aPTT prolongation (n=4)
Leukopenia
Anemia, lymphopenia, neutropenia
Leukopenia, eosinophilia
Anemia Anemia Anemia
Fever
Fatigue, malaise, headache
Fatigue, headache, myalgia
Fever, headache
Fever, headache
Fatigue, headache, myalgia, nausea
RSV
NR NR
NR
Ad26-Ad5 SARS-CoV-2 Phase III, non-randomized, 21977 NR DVT (n=1) NR NR Fever, headache, single-center (72) fatigue, myalgia
The reference numbers preceded by an S refer to references listed in the Online Supplementary Material.The adverse events reported are related only to the vaccinated groups and not to the placebo groups.MERS:Middle East respiratory syndrome virus;SARS-CoV-2:severe acute respiratory syndrome coronavirus 2;RSV:respiratory syncytial virus;HIV:human immunodeficiency virus; NR: not reported; DVT: deep vein thrombosis; PE: pulmonary embolism; CSVT: cerebral sinus venous thrombosis; aPTT: activated partial thromboplastin time; WBC: white blood cell.
3038
haematologica | 2021; 106(12)