Page 26 - 2021_09-Haematologica-web
P. 26
R. Svanberg et al.
low-up, as well as differences between cohorts of patients. Increased T-cell numbers were observed during the first 6 months of treatment in one study,35 while a decrease and normalization of T-cell numbers were found in studies with longer follow-up.33,34,36 This may suggest a correlation between T-cell dynamics and CLL tumor burden during ibrutinib treatment. It was previously demonstrated that T-cell receptor repertoire diversity increased in patients upon ibrutinib treatment, which correlated with disease response and lower infection rates.34 Interestingly, an increase in clonal T cells during ibrutinib treatment, which could be linked to residual CLL disease persistence and the co-occurrence of anti-CLL T-cell clones, was reported recently,37 suggesting that residual disease may maintain certain, specific anti-CLL T-cell clones. Thus, reduced tumor burden as an indirect effect of ibrutinib likely con- tributes significantly to normalization of the majority of
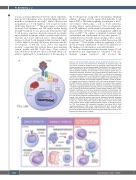
A
the T-cell repertoire along with T-cell numbers. Ibrutinib exhibits off-target activity against IL-2-inducible T-cell kinase (ITK), a TEC kinase signaling downstream of the T- cell receptor, which plays a role in T-cell activation, cytokine release, and proliferation.38 The second-genera- tion BTK inhibitors, acalabrutinib and zanubrutinib, have increased BTK selectivity but an insignificant inhibitory effect on ITK.39,40 In contrast to ibrutinib, treatment with acalabrutinib and zanubrutinib did not alter patients’ T- cell numbers; however, the follow-up time in these studies was limited to 6-7 months, when residual disease may still be present.35,40 Thus, further studies are warranted to clar- ify the potential contribution of direct ITK inhibition to the changes in T-cell numbers seen with ibrutinib.
It was also demonstrated that ibrutinib restored T-cell proliferation and degranulation,36 enhanced T-cell lytic immune synapse function,41 and reversed the
Figure 1. Overview of targets within the chronic lymphocytic leukemia cell, and mechanisms of tumor microenvironment modulation by targeted agents. (A) The chronic lymphocytic leukemia (CLL) cell including targets within the B-cell receptor pathway and anti-apoptotic pathway. Downstream of the B-cell receptor, BTK is inhibited by ibrutinib, acalabrutinib, and zanubrutinib, and PI3Kδ is inhib- ited by idelalisib, duvelisib, and umbralisib. The anti-apoptotic protein BCL-2 is inhibited by venetoclax. (B) Direct versus indirect effects of targeted agents, and activation of tumor microenvironment (TME) anti-CLL activity by novel treatment modalities. Inhibition (both on- and off-target) of targets within the specific TME cells are here referred to as direct effects, exemplified by off-target inhibition of ITK in T cells by ibrutinib, and (on-target) inhibition of PI3Kδ in T cells by idelalis- ib. Changes occurring due to elimination of CLL cells and/or disruption of critical CLL-TME interaction pathways are here referred to as indirect effects, exempli- fied by CLL tumor-debulking by ibrutinib, idelalisib, or venetoclax, and disruption of protective signaling between nurse-like cells/tumor-associated macrophages and CLL cells by ibrutinib and idelalisib. Chimeric antigen receptor (CAR) T cells, bispecific antibodies, and immune checkpoint blockade immunotherapy rely directly on the engagement and activation of microenvironmental cells for anti- CLL activity. Binding of CAR T cells to CD19 on CLL cells activates cytolytic anti- CLL T-cell activity, bispecific antibodies redirect T cells into CLL cell proximity and engage T-cell anti-tumor activity, and immune checkpoint blockade abrogates checkpoint inhibitory signals unleashing the anti-CLL activity of tumor-infiltrating T cells. CLL: chronic lymphocytic leukemia; BTK: Bruton tyrosine kinase; SYK: spleen tyrosine kinase; PI3Kδ: phosphoinositide-3-kinase δ, BCR: B-cell recep- tor; TCR: T-cell receptor; ITK: interleukin-2-inducible T-cell kinase BCL-2: B-cell leukemia/lymphoma-2; TME: tumor microenvironment; NLC: nurse-like cells; TAM: tumor-associated macrophages; CAR T: chimeric antigen receptor T cells; CD: cluster of differentiation; PD-1: programmed cell death protein-1; PD-1L: pro- grammed cell death protein-1 ligand.
B
2314
haematologica | 2021; 106(9)