Page 215 - 2021_09-Haematologica-web
P. 215
Letters to the Editor
(ELISA; R&D Systems, Minneapolis, MN, USA). In the population-based cohort, levels were measured as previ- ously described.6 Measurements were performed inde- pendently in different laboratories. The median pre-treat- ment sCD163 values in the respective cohorts were defined a priori as a cutoff for testing prognostic implica- tions.
In the trial cohort, CD163 mRNA levels in 54 tumor
samples were measured with NanoString nCounter (Nanostring Technologies, Seattle, WA, USA).11 Proportions of CD163+ cells in 41 tumor samples were analyzed by multiplex immunohistochemistry11 (Online Supplementary Figure S1A, B). Blood monocyte counts were available for 103 patients.
Statistical analyses were performed with IBM SPSS Statistics v.25.0 (IBM, Armonk, NY, USA) and STATA/IC
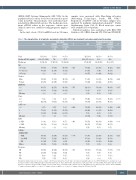
Table 1. The characteristics of all patients and patients divided by sCD163 pre-treatment levels above and below the median.
All
119 (100) 1160 (370-3621) 56 (21-65)
74 (62) 45 (38)
83 (70) 36 (30) 0 (0)
7 (6) 112 (94)
9 (7) 71 (60) 39 (33) 0 (0)
10 (8) 109 (92) 0 (0)
48 (40) 37 (31) 16 (14) 18 (15)
Trial cohort sCD163 sCD163 < median, ≥ median, n(%) n(%)
P
0.519
0.031 0.735
0.233 0.842a
0.491
0.004c
0.315
All
125 (100) 2950 (870-30000) 67 (26-87)
36 (29) 20 (16) 69 (55)
70 (56) 55 (44)
57 (46) 68 (54)
Population-based cohort
sCD163 sCD163
< median, ≥ median, n(%) n(%)
P
0.126
0.644 0.095
<0.001 0.042b
0.009
0.321c
0.013
Total
Median sCD163 (ng/mL) Median age
Age
<60 years 60–65 years >65 years
Gender Male Female
ECOG PS 0-1
2-3
Missing Stage
1-2
3-4
aaIPI score
0-1
2
3 Missing
LDH
≤ ULN > ULN Missing
Subtype DLBCL NOS
GCB non-GCB ND
Other Response
CR PR PD NA
59 (50) 789
57 (21-65)
37 (63)
22 (37)
0(0) 0(0) 0(0)
78 (65) 41 (35)
19 (32)
63 (50) 4610 69 (30-87)
13 (21) 11 (17) 39 (62)
34 (54) 29 (46)
60 (50) 1707 55 (22-64)
41 (68)
62 (50) 2050 64 (26-85)
23 (37) 9 (15) 30 (48)
36 (58) 26 (42)
58 (94)
2 (3)
3(2) 2(3) 1(2)
31 (53) 28 (47)
42 (71) 17 (29) 0 (0)
5 (9) 54 (91)
4 (7) 36 (61) 19 (32) 0 (0)
6 (10) 53 (90) 0 (0)
32 (54) 13 (22) 7 (12) 7 (12)
13 (22)
5(4) 3(5) 2(3)
43 (72) 17 (28)
41 (68) 19 (32) 0 (0)
2 (3) 58 (97)
5 (9) 35 (58) 20 (33) 0 (0)
4 (7) 56 (93) 0 (0)
16 (27) 24 (40) 9 (15) 11 (18)
47 (78) 8 (13) 4(3) 1(2) 3(5)
112 (90) 10 (8)
54 (86) 8 (13)
72 (58) 28 (22)
39 (63) 23 (37)
42 (68)
18 (29) 45 (71)
30 (48)
18 (29) 10 (16)
30 (48)
17 (28)
3(2) 2(3) 1(1)
107 (86) 57 (92) 50 (79) 9(7) 5(8) 4(6) 6(5) 0(0) 6(10) 3(2) 0(0) 3(5)
10 (16)
7(6) 2(3) 5(8)
18 (14)
8 (13)
43 (69)
73 (59) 49 (39)
32 (51)
27 (43)
16 (26)
16 (26)
7(5) 3(5) 4(6)
50 (40) 37 (30) 31 (25)
23 (37) 21 (33) 15 (24)
89 (75) 21 (18)
42 (72)
The patients’characteristics are shown,according to sCD163 pre-treatment levels,in the trial cohort and in a population-based cohort.ECOG PS:Eastern Cooperative Oncology Group performance status; aaIPI: age-adjusted International Prognostic Index; LDH: lactate dehydrogenase; ULN: upper limit of normal; DLBCL NOS: diffuse large B-cell lym- phoma not otherwise specified; GCB: germinal center B-cell like; ND: not determined; CR: complete response; PR: partial response; PD: progressive disease; NA: not available. aComparison between aaIPI score 2 and 3. bComparison between aaIPI score 0-1 and 2. cComparison between GCB and non-GCB.
haematologica | 2021; 106(9)
2503