Page 96 - 2021_06-Haematologica-web
P. 96
M. Swaminathan et al.
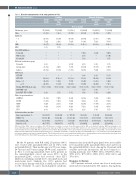
Table 1. Baseline characteristics of all study patients (n=73).
Patients’ characteristics
Median age, years
Male ECOG-PS
0-1
2 AMLa MDS
Prior FLT3 inhibitors
Overall (n=34)
72 [58-82]
20 (59)
27 (79) 7 (21) 33 (97)
Frontline Quizartinib/ AZA (n=15)
75 [64-82]
9 (60)
13 (87) 2 (13) 14 (93)
Quizartinib/LDAC (n=19)
Overall (n=39)
Relapsed/Refractory Quizartinib/ AZA
(n=25)
59 [24-76]
13 (52)
20 (80) 5 (20) 25 (100)
Quizartinib/ LDAC (n=14)
70 [54-84]
8 (57)
9 (64)
5 (36) 14 (100)
70 [58-80]
11 (58)
14 (74) 5 (26) 19 (100)
65 [24-84]
21 (54)
29 (74) 10 (26) 39 (100)
N (%) or [range]
1(3) 1(7) 0 0 0 0
0 0 0 7 (18) 3 (12) 4 (29) 0 0 0 2 (5) 0 2b (14)
Sorafenib
Midostaurin
Crenolanib 0 0 0 1(3) 0 1(7)
ELN risk stratification groupc Favorable
Intermediate
Adverse
2(6) 0 2(11) 2(5) 1(4) 1(7)
FLT3 status FLT3-WT FLT3-ITD+ Ratio<0.5
0
34 (100)
0
19 (100)
17 (68) 7 (28)
2 (8)
22 (88) 10(40)
12 (48) 0.72 [0.01-3.7] 1 (4) 1(4)
8 (57) 5 (36)
1d (7)
13 (93) 6(43)
7 (50) 0.69 [0.07-1.2] 0
2 (14)
3 (25) 3(25) 2 (17) 4(33)
11 (32) 21 (62)
22 (65) 0.71 [0.01-5.4]
2(6)
11 (32)
10(29) 5(33)
9 (27) 2 (13) 6(18) 2(13)
2(6) 0
4 (27) 11 (73)
0
15 (100)
12(35) 5(33) 7(37)
7 (37) 10 (53)
25 (64) 12 (31)
3 (8)
35 (90) 16(41)
19 (49) 0.69 [0.01-3.7] 1 (3) 3(8)
12 (36)
4(12) 1(4)
12 (36) 10 (48) 7(21) 3(14)
≥ 0.5
Median FLT3-ITD allelic ratio FLT3-TKD+ only BothFLT3-ITD+ &TKD+
10 (67) 0.86 [0.02-5.4]
1(7)
12 (63) 0.62 [0.01-2.4]
1(5)
4 (21) 5(26) 7 (37) 4(21) 2(11)
60f [17-97] 3.3 [1.1-21] 9 [7-11.4]
31 [7-75]
Other frequent mutationse
DNMT3A RUNX1 NPM1 TET2 TP53
Laboratory values, median
Bone marrow blasts, % WBC, 109/L Hemoglobin, g/dL
Platelets, 109/L
7 (47)
9 (43)
2(6) 1(4) 1(7)
56 [15-97] 6.1 [1.1-42] 9.1 [7-11.4]
34 [7-377]
56 [15-84] 8 [1.2-42] 9.1 [7.7-10]
54 [14-377]
65 [6-98]
5.3 [0.5-61]
9.4 [7.6-12.8]
25 [4-454]
70 [6-98]
5.2 [0.5-53.4]
9.5 [7.6-12.7]
22 [4-99]
49 [21-86]
5.4 [0.9-61]
9.2 [7.7-12.8]
25 [12-454]
AZA: azacitidine; LDAC: low-dose cytarabine; ECOG-PS: Eastern Cooperative Oncology Group-Performance Status; AML: acute myeloid leukemia; MDS: myelodysplastic syn- dromes;ELN:European LeukemiaNet;ITD:internal tandem duplication;TKD:tyrosine kinase domain;WBC:white blood cells.aSix patients in the AZA and 12 in the LDAC cohort treated frontline had secondary AML.Three patients each in both the AZA and LDAC cohorts given with first salvage therapy had secondary AML.bOne patient who received LDAC was treated with both sorafenib and midostaurin prior to quizartinib. cClassified according to the European LeukemiaNet 2017. dOne patient had a prior FLT3-TKD muta- tion that was negative at the time of starting treatment.eThe 81-gene panel was not used for mutational analysis prior to starting treatment in four patients given first salvage treat- ment with AZA and two given LDAC. fTwo patients were diagnosed based on circulating blast percentages of 22% and 25%.
Of the 39 patients with R/R AML, 25 patients (64%) were treated with quizartinib/AZA and 14 (36%) with quizartinib/LDAC. Their median age was 59 years (range, 24-76 years) and 70 years (range, 54-84 years), respective- ly. Prior to receiving quizartinib, three (12%) patients in the quizartinib/AZA cohort and seven (50%) in the quizartinib/LDAC cohort had received other FLT3 inhibitors, including sorafenib (n=7), crenolanib (n=2), and midostaurin (n=1). Similarly, five (13%) patients had received prior therapy with AZA for AML and four (10%) for the treatment of a prior myelodysplastic syndrome.
Mutation data (from the 81-gene panel) were not available for four patients in the quizartinib/AZA cohort and two in the quizartinib/LDAC cohort. Among the 33 patients with available molecular information, the most common co- existing mutations were NPM1 (36%), DNMT3A (36%), TET2 (21%), WT1 (15%), IDH2 (15%), IDH1 (12%), and TP53(6%).
Response to therapy
All 73 patients received at least one dose of study treat- ment and were included in the ITT analysis. Among them,
2124
haematologica | 2021; 106(8)