Page 226 - 2021_06-Haematologica-web
P. 226
Letters to the Editor
AC
D
BE
G
F
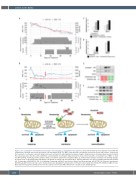
Figure 2. The combination of ibrutinib and venetoclax is clinically active in T-prolymphocytic leukemia. (A, B) Clinical follow-up of two patients treated with the combination of ibrutinib and venetoclax, patient A (A) and patient B (B). The WBC count and LDH concentration are plotted as blue and red lines, respectively. The lower part of each panel represents drug serum levels as black dots and drugs given as gray rectangles. Drug levels were determined by mass spectrometry. The red arrow denotes the time point at which the serum ibrutinib concentration dropped below the level of detection with a concomitant rise of WBC. (C, D) In vivo BH3 profiling of primary patients’ samples during co-treatment: patient A (C) and patient B (D). (E, F) Western blot analysis of primary cells showing changes in protein levels of ITK and phospho-ITK during co-treatment of patient A (E) and patient B (F). Primary antibodies were directed against phospho-ITK (Tyr512, Life Technologies, #PA564523), ITK (Cell Signaling Technology, #2380S) and b-actin (Santa Cruz Biotechnology, #SC-47778) (G) Proposed mechanism. Monotherapy with venetoclax may lead to drug resistance via upregulation and activation of ITK and reduced apoptotic priming. ITK inhibition might increase Bcl-2-dependent apoptotic priming and restore the activity of venetoclax. WBC: white blood cell; LDH: lactate dehydrogenase.
2254
haematologica | 2021; 106(8)