Page 13 - 2021_07-Haematologica-web
P. 13
Editorials
JAK out of the box: myeloproliferative neoplasms--associated JAK2 V617F mutations contribute to aortic aneurysms
Shannon E. Elf
The Ben May Department for Cancer Research, The University of Chicago, Chicago, IL, USA E-mail: SHANNON ELF - shannonelf@uchicago.edu
doi:10.3324/haematol.2020.277111
Myeloproliferative neoplasms (MPN) are clonal dis- orders of hematopoiesis arising in the hematopoietic stem cell (HSC) compartment and characterized by the excess production of mature myeloid cells.1 BCR-ABL-negative MPN include polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF). PV is characterized by uncontrolled red blood cell production; ET, by megakaryocytic hyperplasia and elevat- ed platelet counts; and MF, by megakaryocytic hyperplasia and bone marrow fibrosis. The molecular basis of MPN remained unknown until 2005, when four different groups described a point mutation in the pseudokinase domain of Janus kinase-2 (JAK2), a non-receptor tyrosine kinase, in the majority of MPN patients. The resulting JAK2 V617F mutant protein was found to be constitutively active, lead- ing to hyperactive JAK-STAT signaling downstream of multiple hematopoietic cytokine receptors.2-5
Although MPN, particularly PV and ET, are commonly characterized as “indolent” diseases, patients have signif- icantly decreased life expectancies compared to the gen- eral population. JAK2 V617F mutations are associated with increased vascular complications, which to date pri- marily include venous and arterial thrombosis and advanced atherosclerosis.6,7 Indeed, fatal thrombotic events represent the leading cause of death in JAK2
V617F-positive MPN patients, though the underlying mechanisms remain elusive. Mouse models of Jak2 V617F-driven MPN faithfully recapitulate this phenotype, with lethality primarily attributed to thrombosis.8
Interestingly, the prevalence of the JAK2 V617F muta- tion has also been found to be significantly increased in non-MPN patients with coronary artery disease and peripheral artery disease,9,10 suggesting that the JAK2 V617F mutation may play a role in additional vascular diseases. This observation is supported by the phenome- non of clonal hematopoiesis, in which clonal expansion of hematopoietic cells carrying is associated with signifi- cantly increased risk of vascular disorders.11 However, the mechanisms underlying JAK2 V617F-mediated vascular disease remain unclear, and the association of JAK2 V617F with vascular diseases outside of thrombosis, ath- erosclerosis, coronary artery disease and peripheral arteri- al disease has yet to be studied.
In this issue of Haematologica, Yokokawa et al.12 inves- tigate the contribution of bone marrow (BM)-derived JAK2 V617F to the development of aortic aneurysms (AA), a vascular disease not yet studied in MPN patients. AA often progress asymptomatically and can lead to sud- den death, so understanding the mechanisms underlying their onset and associated risk factors is critically impor-
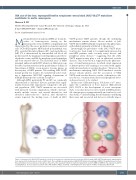
Figure 1. Hematopoietic JAK2 V617F mutations lead to the development of abdominal aortic aneurysms (AAA). Somatic JAK2 V617F mutations are acquired in the bone marrow at the hematopoietic stem cell (HSC) level. The resulting bone marrow-derived JAK2 V617F-mutated macrophages infiltrate the abdominal aorta, where they demonstrate increased levels of two genes critical for aortic aneurysm formation, matrix metalloproteins 2 and 9 (MMP-2, MMP-9). This expression and the resulting AAA are decreased upon treatment with a JAK inhibitor (JAK2i), ruxolitinib, confirming that the development of AAA is mediated by JAK2 V617F.
haematologica | 2021; 106(7)
1783