Page 48 - 2021_06-Haematologica-web
P. 48
C. Zhang et al.
A
BC
D
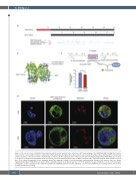
Figure 2. The short isoform of ALDH1A2 has enzymatic activity and is localized in the cytoplasm. (A) Protein domains of the ALDH1A2. NAD+ binding site, homote- tramer interface and catalytic residues are indicated individually by blue triangles. The truncated short isoform lacks 96 amino acids at the N-terminus. The deleted tetramer interface of the short isoform is marked with a red block. (B) The crystal structure of the homotetrameric ALDH1A2 long isoform (PDB: 4X2Q) is presented as a cartoon model generated by PyMOL. Each monomer is colored in a different shade of green. Within a monomer, the region absent in the short isoform is colored blue. (C) A scheme showing the in vitro enzymatic assay. The enzymatic activity of each isoform was evaluated by the velocity of the kinetics curve (see Online Supplementary Figure S3B). Error bars represent the standard deviation for technical replicates. *P<0.05 using the two-tailed Student t test. (D) Confocal images showing the cellular localization of the endogenous EGFP-fused ALDH1A2 proteins in Jurkat and K562 cells. Hoechst and Mitotracker staining evidence the nucleus and mitochondria, respectively.
1550
haematologica | 2021; 106(6)