Page 19 - 2021_06-Haematologica-web
P. 19
Role of the Hsp70 chaperone in erythropoiesis
required for efficient Hb biogenesis. Heme synthesis is mediated by conjugating iron and protoporphyrin in a series of enzymatic reactions occurring in mitochondria and cytosol.24 The hydroxyl radicals produced by elevated levels of free heme/iron undergoing the Fenton reaction in erythroblast cytosol could damage and induce the aggregation of both Hb and other critical biomolecules.25
A
The next PQC challenge in red blood cell maturation occurs from the reduction/disruption of crucial PQC pathways that typically protect cells against protein aggregation. In order to make space for the increasing lev- els of Hb, the proteome of the terminally differentiating erythroblasts is rapidly reduced to 2-5% via bulk degra- dation of many cellular proteins and organelles3 by the
B
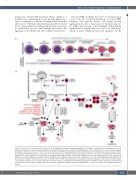
Figure 1. Biogenesis of hemoglobin during erythropoiesis. (A) A simplified schematic diagram showing the key cell stages of erythropoiesis. Hematopoietic stem cells (HSC) differentiate into a common myeloid progenitor, which further transition into a committed erythroid lineage. The proerythroblast is the earliest morphologically identifiable erythroid precursor cell in the bone marrow. Erythropoietin (EPO) signaling initiates terminal differentiation of erythroblasts to generate mature erythro- cytes. During terminal differentiation, cells reduce in size and undergo major changes including chromatin condensation, proteome remodeling and ultimately the elimination of cellular organelles to provide room for hemoglobin (Hb). Hb expression levels in differentiating cells are indicated by intensity of the red color. (B) Chaperone assisted folding and assembly of Hb. The folding of nascent globin chains is assisted by chaperones such as the α-Hb–stabilizing protein (AHSP), heat shock protein 70 (Hsp70) and heat shock protein 90 (Hsp90). Errors in Hb subunit synthesis and assembly, iron/heme imbalances, deficiencies in protein quality control activities and exposure to reactive oxygen species (ROS), however, could trigger the misfolding and aggregation of globin proteins. In particular, misfolded and unassembled α-globin chains are highly prone to form cytotoxic aggregates leading to ineffective erythropoiesis. c: cytosol; n: nucleus; UPS: ubiquitin proteasome system.
haematologica | 2021; 106(6)
1521