Page 179 - 2021_06-Haematologica-web
P. 179
Mobilization regulated by PPARd
AB
CD
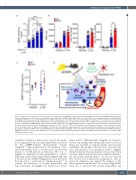
Figure 7. Angptl4 in bone marrow as a negative regulator of mobilization. (A) Angptl4 protein level in bone marrow (BM) extracellular fluid (BMEF) during granulocyte colony-stimulating factor (G-CSF)-induced mobilization with or without the PPARd agonist GW501516 (n=3). (B) G-CSF-induced mobilization in mice treated with the anti-Angptl4 antibody as assessed by white blood cells (WBC), lineage-Sca-1+c-kit+ (LSK) cells and colony-forming units in culture (CFU-C) in the blood (n=3 for group treated with phosphate-buffered saline [PBS]/bovine serum albumin [BSA] and n=7 for group treated with G-CSF). (C) BM vascular permeability assessed by Evans blue dye concentration in BMEF during G-CSF mobilization with or without the anti-Angptl4 antibody (n=3-8). Combined data from at least three independent exper- iments are shown. Data are mean ± standard error of mean. *P<0.05, **P<0.01, ***P<0.001 (Student t test and analysis of variance). (D) Schematic representa- tion of the proposed role of dietary fat and PPARd in G-CSF-induced mobilization. High sympathetic tone induced by G-CSF stimulates b1/b2-adrenergic receptors (b1/b2 AR) to upregulate PPARd expression in BM myeloid cells. Dietary fatty acid ligands, such as ω3-poyunsaturated fatty acids (PUFA), bind to PPARd and promote Angptl4 expression to suppress the mobilization via, at least partially, inhibition of BM vascular permeability. The PPARd-independent pathway of ω3-PUFA to inhibit mobilization may also exist.
sensitivity, and glucose homeostasis. Second, the regula- tion of angiogenesis and vascular permeability is mediated by the COOH-terminal fibrinogen-like domain (cAngptl4).23,24 Among these effects, the regulation of vas- cular permeability seems to be the most relevant with respect to G-CSF-induced mobilization. The role of Angptl4 in regulating vascular permeability is context- dependent. Early studies suggested that Angptl4, although it was not shown whether cAngptl4 was used, decreased the leak of dye or extravasation of melanoma cells.39,40 In contrast, the promotion of vascular permeability and tumor metastasis by Angptl4 was reported in a breast
tumor model.41 Mechanistically, cAngptl4 was shown to activate α5b1 integrin and subsequently decluster VE-cad- herin and claudin-5 in primary human microvascular endothelial cells, leading to the induction of vascular leak- iness and metastasis in a melanoma model.42 Angptl4- mediated increased vascular leakiness was also reported in nontumor pathological models such as influenza pneumo- nia and diabetic macular edema.25,43 It was also reported that altered post-translational modification, such as decreased sialylation, can augment the leakiness of the kidney glomerular epithelium.44 In our study, Angptl4 inhi- bition led to increased BM vascular permeability and
haematologica | 2021; 106(6)
1681