Page 172 - 2021_05-Haematologica-web
P. 172
O. Moser et al.
matoses, Fanconi anemia. There was no systematic screening for CPC in the study population. Reported patients were identified by the presence of clinical immune deficiency, and/or signs of the syndromes stated above. Investigations for CPC were prompted by multi- ple-cancer development and/or diminished therapy toler-
ance in some cases. Six of 57 patients with CPC received radiotherapy. Altogether, 24.7% of all 3,590 patients received ALL-type therapy and 75.3% B-NHL-type regime. 450 patients in the analyzed cohort (12.5%) suf- fered from relapse of the NHL. Treatment for relapsed dis- ease consisted of individualized chemotherapy. Two hun-
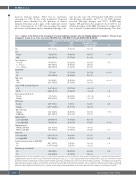
Table 1. Analysis of risk factors for the development of second malignant neoplasms after non-Hodgkin lymphoma in children < 15 years of age at diagnosis, treated in one of the consecutive NHL-BFM studies NHL-BFM-81, through EURO-LB-02/B-NHL-04.
Risk factors for development of SMN, univariate analysis
All patients N (%)
3590 (100%)
952 (26.5%)
2,638 (73.5%)
663 (18.5%) 1,495 (41.6%) 1,432 (39.9%)
57 (1.6%)
3,533 (98.4%)
810 (21.2%) 2,780 (77.4%)
1,008# (29.0%)
2,464# (71.0%)
705 (19.6%) 2,885 (80.4%)
249## (7.3%)
3,170## (92.7%)
1,120 (31.2%) 2,470 (68.8%)
2,944 (82.0%)
646 (18.0%)
2,160 (60.2%) 1,430 (39.8%)
1,935* (72.0%)
753* (28.0%)
486### (13.7%) 3,066### (86.3%)
524 (14.6%)
3,066 (85.4%)
SMN N (%)
95 (100%)
40 (42.1%)
55 (57.9%)
21 (22.1%) 43 (45.3%) 31 (32.6%)
11 (11.6%)
84 (88.4%)
41 (43.2%) 54 (56.8%)
17 (17.9%)
78 (82.1%)
22 (23.2%) 73 (76.8%)
9 (9.5%)
86 (90.5%)
47 (49.5%) 48 (50.5%)
63 (66.3%)
32 (33.7%)
77 (81.1%) 18 (18.9%)
39 (81.3%)
9 (18.7%)
7 (58.3%) 5 (41.7%)
24 (25.3%)
71 (74.7%)
Cumulative incidence
at 20 years (SE) P
All
Sex
Female
Male
Age at diagnosis <5 years 5–10years >10 – 14 years
Known CPC
Yes
No
NHL entity LBL
other
Stage of NHL at primary diagnosis
I, II
III, IV
Bone marrow involvement Positive
Negative
CNS status
Positive
Negative
Type of therapy ALL-type B-NHL-type
5.3 (0.7)
8.4 (1.7)
4.0 (0.8)
0.0008
6.1 (1.8) 0.51 5.4 (1.2)
4.6 (1.1)
19.9 (7.2)
4.9 (0.7)
9.3 (1.9) 3.9 (0.8)
0.03 (0.01)
<0.0001
<0.0001
0.1 (0.04) 0.08
0.07 (0.02) 0.61 0.05 (0.01)
3.9 (1.7) 0.21
Anthracyclines+
5.4 (0.8)
8.7 (1.7) 3.7 (0.7)
4.3 (0.8)
8.7 (1·8)
0.0002
<0.0001
≤ 200 mg/m2 BSA
> 200 mg/m2 BSA
Alkylating agents++ CPM≤2g/m2BSA CPM>2g/m2BSA
> 800 mg/m2 BSA
Cranial radiotherapy/risk for CNS-SMN Yes
No
Radiotherapy (overall risk)
Yes
No
0.8 (0.6)
5.3 (2.4) 0.30
3.5 (0.7)
1.6 (0.9) 0.94
1.9 (0.8) 0.01 0.2 (0.1)
4.9 (1.2) 0.86
5.2 (0.9)
Etoposide
≤ 800 mg/m2 BSA
Cumulative drug doses were calculated as per protocol. +Doxorubicin equivalent dose was calculated at 1:1 ratio for the used anthracyclines (doxorubicin and daunorubicin). ++Alkylating agents: conversion factor for cyclophosphamide equivalent dose was 1:4 (i.e., 1 mg cyclophosphamide equals 4 mg ifosfamide); *indicates only patients receiving B-NHL-type therapy; # information about stage of disease missing in 118 patients; ##information about CNS involvement missing in 171 patients; ###information about cranial radio- therapy missing in 38 patients. SMN: second malignant neoplasm; NHL: non-Hodgkin lymphoma; ALCL: anaplastic large-cell lymphoma; ALL: acute lymphoblastic leukemia; B- NHL: mature B-cell lymphoma; BSA: body surface area; CI: Confidence Interval; CNS: central nervous system; CPC: cancer-predisposing condition, CPM: cyclophosphamide; HR: hazard ratio; LBL: lymphoblastic lymphoma; nfc: not further classified; NHL: non-Hodgkin Lymphoma; N: number; SE: standard error; SMN: second malignant neoplasm.
1392
haematologica | 2021; 106(5)