Page 64 - 2021_04-Haematologica-web
P. 64
J.M. Logue et al.
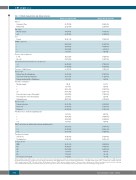
Table 1. Patient characteristics and clinical outcomes.
Axi-cel
Standard of Care Clinical trial
Age, years
Analysis up to day 30 (n=85)
63 (74.1%) 22 (25.9%)
64 (28-79)
40 (47·1%)
34 (40·0%)
28 (32·9%) 38 (44·7%) 16 (18·8%) 3 (3·5%)
18 (21·2%) 67 (78·8%)
28 (32·9%)
57 (67·1%) 20 (23·5%)
56 (65·9%) 26 (30·6%) 3 (3·5%)
3 (1-8) 3 (3·5%) 26 (30·6%) 56 (65·9%) 21 (24·7%) 2 (2·4%) 48 (56·5%)
26 (30·6%) 31 (36·5%) 28 (32·9%)
6 (7·1%) 36 (42·4%) 35 (41·2%) 8 (9·4%)
28 (32·9%) 31 (36·5%) 26 (30·6%)
39 (45·9%) 39 (45·9%)
68 (80·0%) 41 (48·2%) 27 (31·8%) 9 (10·6%) 7 (8·2%)
1 (1·2%)
Analysis after day 30 (n=70)
48 (68.6%) 22 (31.4%)
63 (28-79)
29 (41·4%)
27 (38·6%)
26 (37·1%) 34 (48·6%) 9 (12·9%) 1 (1·4%)
17 (24·3%) 53 (75·7%)
27 (38·6%)
43 (61·4%) 13 (18·6%)
47 (67·1%) 20 (28·6%) 3 (4·3%)
3 (1-7)
3 (4·3%) 25 (35·7%) 42 (60·0%) 17 (24·3%) 2 (2·9%) 36 (51·4%)
22 (31·4%) 25 (35·7%) 23 (32·9%)
4 (5·7%) 34 (48·6%) 29 (41·4%) 3 (4·3%)
25 (35·7%) 28 (40·0%) 17 (24·3%)
27 (38·6%) 28 (40·0%)
64 (91·4%) 38 (54·3%) 26 (37·1%) 6 (8·6%)
0 (0·0%)
0 (0·0%)
Median (range)
≥65 Sex
Female
ECOG at apheresis
0 1 2 3
Disease stage at apheresis IorII
III or IV
International Prognostic Index score at apheresis
0-2
3-5
Presence of bulky disease Disease type
Diffuse large B-cell lymphoma Transformed follicular lymphoma Primary mediastinal B-cell lymphoma
Prior lines of therapies Median (range)
1
2
≥3
Prior autologous stem cell transplant Prior allogeneic stem cell transplant Bridging therapy
Disease status Primary refractory Refractory Relapsed
Cytokine release syndrome maximum grade 0
1 2 3-4
CAR T-cell related encephalopathy syndrome maximum grade
0 1-2 3-4
Treatment for toxicity Steroid use Tocilizumab use
30-day response
ORR
CR
PR
SD
PD
No response assessment
Patient baseline characteristics prior to axi-cel and clinical outcomes following axi-cel infusion for patients analyzed prior to and after day 30. axi-cel: axicabtagene ciloleucel; IQR: interquar- tile range; ECOG: Eastern Cooperative Oncology Group performance status; Bulky disease: any mass diameter greater than 10 cm; ORR: overall response rate (calculated as complete response [CR] + partial response [PR]); SD: stable disease; PD: progressive disease; CAR T: chimeric antigen receptor T . Disease status at apheresis was defined as: primary refractory, never achieving end of treatment CR; refractory, not primary refractory and no response to the most recent therapy; relapsed, responded to most recent therapy and progressed.
980
haematologica | 2021; 106(4)