Page 33 - 2021_04-Haematologica-web
P. 33
Bioengineering approaches to blood cell production
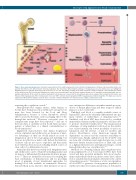
Figure 1. Bone marrow hematopoiesis. Schematic representation of the adult hematopoietic stem cell niche, showing various cell types and extracellular matrix com- ponents that influence the differentiation of blood progenitors. The hierarchical differentiation pathways of megakaryopoiesis and erythropoiesis are highlighted. Megakaryopoiesis is typically characterized by an increase in cell size and ploidy, resulting in the final extension of long pseudopods, called proplatelets, which release platelets into the bloodstream. Erythropoiesis entails several morphological and structural changes that give rise to basophilic, polychromatophilic and aci- dophilic erythroblasts. At the end of the terminal maturation reticulocytes are released into the bloodstream where they complete their maturation into mature ery- throcytes. Mk: megakaryocyte; HSC: hematopoietic stem cell; CMP: common myeloid progenitor; MEP: megakaryocyte-erythroid progenitor. The figure was created using Servier Medical Art templates licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
activating the coagulation cascade.37
Hemoglobin-based oxygen carriers, either human or
bovine, have been proposed as erythrocyte surrogates, but none has been licensed by the Food and Drug Administration because of severe thrombotic adverse effects caused by the nitric oxide-scavenging effect of the hemoglobin molecule.38 However, successful cases of compassionate usage have been reported,39,40 and one of these products is currently used in South Africa in emer- gencies or when there is a clinical contraindication to blood transfusion.41
Engineered nanostructures that mimic biophysical actions of platelets and erythrocytes are therefore of inter- est. Advantages of their use would include no need of refrigeration and of blood grouping and matching, but they are not effective as native cells. Indeed, the functions of native cells are not easy to reproduce and thus much more effort has been focused on finding reliable sources of stem cells to be differentiated in vitro.
Animal models have been widely used. Megakaryocytes and erythrocytes can be obtained by flushing murine and rat femora or differentiated from murine fetal liver progen- itors.42,43 These are invaluable cell sources for studying the basic mechanisms of hematopoiesis and for providing proof of principle of new translational approaches for making blood cells available for transfusion. However, beyond ethical issues related to their intensive use in sci-
ence, interspecies differences can render animals poor pre- dictors of human physiology and their usage for clinical purposes is not conceivable.
Umbilical cord blood is a rapidly available source of human HSC that can be efficiently differentiated into pri- mary cultures of erythroblasts or megakaryocytes.44,45 Umbilical cord blood HSC have been used to establish immortalized human erythroid progenitor cell lines able to produce enucleated erythrocytes.46 The main practical advantages of using umbilical cord blood are the relative ease of procurement, the lowest possibility of viral con- tamination and the absence of risk for mothers and donors. Nevertheless, active limitations remain the dependence on donors and the restricted availability to research teams. Finally, umbilical cord blood CD34+ cells are stem cells of fetal/neonatal origin that give rise to cells that have distinct features from those of adult cells, such as a high proliferation rate and high percentages of fetal hemoglobin in the case of erythrocytes.47-50
Methods for obtaining human adult megakaryocytes from peripheral blood or bone marrow HSC have been tested and have provided invaluable data about mecha- nisms of platelet production in disease states due to inher- ited or acquired mutations in genes relevant for the control of megakaryopoiesis.51 Giarratana et al. also used peripher- al blood HSC to generate a homogeneous population of erythrocytes that were functional in terms of deformabil-
haematologica | 2021; 106(4)
949