Page 287 - 2021_04-Haematologica-web
P. 287
Letters to the Editor
The cytoplasmic level of a messenger RNA relies not only on its rate of synthesis but also on its decay rate. Therefore, in order to determine if the hepcidin level in response to Tm is regulated through induction of its tran- scription or through increased stability of its mRNA, HepG2 cells were treated with the transcriptional inhibitor actinomycin D. As expected, treatment with actinomycin D significantly reduced hepcidin mRNA lev-
els in these cells (Figure 2E). However, when treated with Tm in the presence of actinomycin D, HepG2 cells still exhibited a significant increase in hepcidin mRNA expression (Figure 2E), clearly demonstrating that ER stress controls hepcidin gene expression through post- transcriptional mechanisms.
Interestingly, ELAVL1/HuR was recently described as a protein stabilizing hepcidin mRNA in response to satu-
ABC
DE
FG
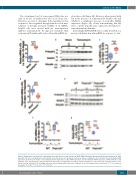
Figure 1. Endoplasmic reticulum stress upregulates hepcidin expression and activates the BMP-SMAD signaling pathway partially through matriptase-2. Wild- type (WT) CD1 mice (6-9/group) were injected with mock (blue boxes) or tunicamycin (red boxes) and were analyzed 6 hours (h) later for liver (A) Hamp mRNA expression; (B) P-Smad 5 relative to total Smad5 protein expression; (C) Id1 mRNA expression and (D) Tmprss6 mRNA expression. C57Bl/6 WT mice and Tmprss6-/- mice (3-4/group) were injected with mock (blue boxes) or tunicamycin (red boxes) and were analyzed 6 h later for liver (E) P-Smad 5 relative to total Smad 5 protein expression; (F) Id1 mRNA expression and (G) Hamp mRNA expression. Estimates of the fold changes in gene expression (2−DDCt) are shown on the graphs. *P<0.05; ***P<0.001; ****P<0.0001.
haematologica | 2021; 106(4)
1203