Page 273 - 2021_04-Haematologica-web
P. 273
Letters to the Editor
F
continued from previous page
G
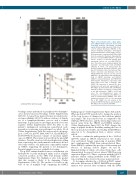
Figure 1. The proband carries a likely patho- genic UBE2T variant expressed at low levels conferring defective interstrand crosslink repair. (A) Sequencing of genomic DNA extract- ed from primary fibroblasts (PM085) of the affected individual indicating a homozygous chr1:202333539G>T variant (hg38, reverse). (B) Sequencing of complementary DNA (cDNA) from the proband’s fibroblasts indicating the presence of a variant NM_014176.3:c.196C>A and no evidence of aberrant splicing. Exon numbering reflects ref seq NM_014176.3 since the primers were designed against this transcript.6 (C) Immunoblot with anti-UBE2T antibody in whole cell extract from the proband’s primary fibroblasts (PM085), wild- type BJ fibroblasts (from the American Type Culture Collection) and fibroblasts from an UBE2T/FANCT-null Fanconi anemia patient (RA2627).6 (D) Immunoblot with anti-HA anti- body in PM085 (proband) and RA2627 (UBE2T-/-) primary fibroblasts and PM085 EH (immortalized fibroblasts) expressing C-HA- FLAG empty vector (EV) or wild-type (WT) UBE2T. HA expression in parental (P) (non- transduced) cells and cells expressing EV or WT UBE2T. (E) Immunoblot with anti-FANCD2 antibody on whole cell extracts of cells treated or not with mitomycin C (MMC). Ub-D2 indi- cates the monoubiquitinated band. (F) Formation of foci of FANCD2 after MMC treat- ment in patient-derived PM085 cells (non- transduced parental cells) or cells expressing EV, or WT UBE2T. (G) Cell survival of the proband’s PM085 fibroblasts expressing EV or WT UBE2T after treatment with MMC.
breakage assays performed on peripheral blood lympho- cytes showed increased breakage (Online Supplementary Table S1). A repeat bone marrow biopsy revealed moder- ate hypocellularity (40-50%) with no evidence of dyspla- sia or a lymphoproliferative process and a normal karyotype. A gene panel to investigate periodic fever was negative (Online Supplementary Table S3). Due to the patient’s undiagnosed neutropenia, panel-based next- generation sequencing was performed on whole blood (Online Supplementary Table S4) and revealed a homozy- gous c.196C>A, p.P66T (NM_014176.3, Chr1(GRCh37): 202302667G>T) missense variant of uncertain signifi- cance in UBE2T. This variant is absent from the gnomAD database. GeneDx exon level deletion/duplication calling from sequencing data (with manual verification) did not detect any evidence of a multi-exon copy number variant in UBE2T, suggesting the patient is not hemizygous. Parental samples were not available for testing.
The p.P66T variant identified causes a substitution of a hydrophobic to polar uncharged amino acid at a highly conserved position in the UBC fold domain (Online Supplementary Figure S1). Multiple in silico tools predict that this variant is likely to be damaging (Online Supplementary Table S5). The proline 66 resides at the base of one of multiple loops comprising the FANCL
binding region10 (Online Supplementary Figure S2A and B). When modeled, P66T is predicted to change the position of the loop because of changes in the backbone phi/psi (φ/ψ) angles. The loop is moved out, as compared to the wild-type (WT) structure, and the interacting residues are moved away from the UBE2T and FANCL interface (Online Supplementary Figure S2C). As P66T changes the range of peptide backbone flexibility, making the base of the loop much more flexible, the binding with FANCL is expected to be dysregulated from a stricter cis/trans switch.
In order to confirm the pathogenicity of the c.196C>A (p.P66T) variant in UBE2T, functional in vitro studies were performed. Sanger sequencing of genomic DNA and complementary DNA from patient-derived fibro- blasts (PM085) confirmed the presence of this variant and absence of splicing defects (Figure 1A and B). Immunoblotting of whole cell extract from these cells demonstrated decreased, but not absent, UBE2T protein expression (Figure 1C). This is consistent with the p.P66T missense variant causing instability in the UBE2T pro- tein, resulting in the observed decrease in protein level.
To determine whether the c.196C>A (p.P66T) variant affects the E2 function of UBE2T, FANCD2 monoubiqui- tination was assessed after treatment with the DNA
haematologica | 2021; 106(4)
1189