Page 182 - 2021_04-Haematologica-web
P. 182
I. Abou Dalle et al.
instances, secondary myeloid malignancies.5,6 There is an unmet need to develop strategies aimed at selectively protecting HSC from the damaging effects of chemother- apy, and maintaining the HSC pool especially for cancer survivors. Growing evidence suggests that the luteiniz- ing hormone/choriogonadotropin receptor (LHCGR) is expressed on human HSC and that LH is implicated in HSC self-renewal.7,8 In preclinical murine models, LH suppression using an LH-releasing hormone (LHRH) antagonist improved hematopoietic recovery after
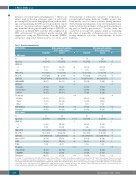
Table 1. Baseline characteristics.
Characteristics Before propensity matching N (%), median [range]
chemotherapy or lethal dose radiation.9-11 Leuprolide, a gonadotropin-releasing hormone (GnRH) agonist, has been widely used in cancer patients during intensive chemotherapy and allogeneic stem cell transplantation in order to reduce the incidence of abnormal uterine bleed- ing and for fertility preservation.12-14 In this study, we conducted a retrospective analysis aimed at evaluating the effect of leuprolide on hematopoietic recovery fol- lowing intensive cytotoxic chemotherapy in acute leukemia.
After propensity matching N (%), median [range]
Leuprolide Control P
N 66 388 64 128
A. AML cohort
Leuprolide
33 [19-54]
Control P
35 [18-56] 0.7 1
Age, years ECOG PS
33 [19-54]
47 [18-56]
<0.001 0.4
6.6 [0.5-390] 0.7
42.0 [1-676] 0.8 920 [200-14,701] 0.1 120 (31) 0.8 0.1
78 (20) 194 (50) 116 (30)
0-1
≥2 6(9) 51(13) 6(9) 11(9)
WBC x109/L Plt x109/L LDH UI/L AMML/AMOL ELN Risk
Favorable Intermediate Adverse
39 (31) 53 (41) 36 (28)
58 (91)
7.1 [0.7-160]
38.5 [3-271] 786 [297-18,336] 22 (33)
21 (32) 27 (41) 18 (27)
326 (87)
58 (91)
7.2 [0.7-160] 37 [3-271] 789 [297-18,336] 22 (34)
21 (33) 26 (41) 17 (26)
117 (91)
6.6 [0.5-80] 0.6
35.5 [2-575] 0.7 886 [322-14,701] 0.1 31 (24) 0.2 0.9
Treatment*
Doublet
Triplet
Other 6(9) 12(3) 6(9) 7(6)
0.04
0.02 14 (22) 10 (9)
0.04 20 (31) 37 (29) 0.7
49 98
Age, years
ECOGPS 1 1
FLT3 Inhibitor Transplant
B. ALL cohort
N
14 (21) 41 (11) 27 (41) 110 (28)
65 192
0.009
23 (35) 37 (56)
205 (53) 171 (44)
22 (35) 36 (56)
54 (42) 67(52)
0.4
31 [18-49] 41 [18-56]
<0.001 32 [18-49] 32 [18-55] 0.6
0-1
HyperCVAD
AugBFM
Transplant
50 (85)
9 (15)
5.8 [0.6-316] 51 [0-495] 1,096 [284-28,015] 32 (54)
58 (89)
7 (11)
43 (66)
18 (28)
24 (37)
145 (84)
28 (16)
5.4 [0.5-420] 29 [2-393] 1,256 [238-37,602] 87 (50)
178 (93)
14 (7)
169 (88)
23 (12)
37 (19)
42 (86) 7 (14)
0.9 7.6 [0.6-316] 0.1 54 [0-495]
0.5 1,109 [284-28,015] 0.6 25 (51)
0.4 44 (90) 5 (10)
85 (87)
13 (13)
7 [0.6-420] 0.5 50 [5-395] 0.8
1,125 [268-37,602] 0.8 47 (48) 0.7
89 (91) 1 9 (9)
0.07
19 (19) 0.02
≥2
WBC x109/L Plt x109/L LDH UI/L Adverse CG** B-ALL
T-ALL Treatment
0.001
34 (69)
13 (27)
83 (85)
15 (15)
0.006 19 (39)
Propensity score matching included all the above baseline characteristics. * Doublet chemotherapy: idarubicin and cytarabine (IA); triplet chemotherapy: IA plus a nucleoside analog (i.e., cladribine, clofarabine, or fludarabine). **Adverse cytogenetics: complex karyotype (≥ 5 abnormalities) t(9;22), t(4;11), and low hypodiploidy/near-triploidy. AML: acute myeloid leukemia; ALL: acute lymphoblastic leukemia; N: number; ECOG PS: Eastern Cooperative Oncology Group performance status; WBC: white blood cell; Plt: platelet count; LDH: lactate dehydrogenase level; AMML/AMOL: acute myelomonocytic leukemia and monocytic leukemia; HyperCVAD: hyperfractionated cyclophosphamide, vin- cristine, adriamycin, dexamethasone; AugBFM: Augmented BFM regimen; ELN: European LeukemiaNet.
1098
haematologica | 2021; 106(4)