Page 172 - 2021_04-Haematologica-web
P. 172
V. Brixner et al.
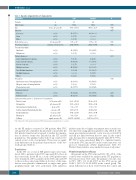
Table 1. Baseline characteristics of study patients. Parameter
UVC
87
55/32 56.67 ± 14.11
85 (97.70) 1 (1.15)
1 (1.15) 1.95 ± 0.20 27/32 (84.38)
86 (98.85) 1 (1.15)
7 (8.05) 40 (45.98) 1 (1.15) 22 (25.29) 9 (10.34) 0 (0.00) 8 (9.20)
29 (33.33) 6 (6.90) 52 (59.77)
55 (63.22)
54 (62.07)
59.05 ± 75.21 91.15 ± 14.04 1.02 ± 0.11 28.66 ± 5.98 97.41 ± 15.49 3.16 ± 1.23 2935.75 ± 4086.45
Control
84
52/32 54.79 ± 11.90
84 (100.00) 0 (0.00)
0 (0.00) 1.97 ± 0.23 23/32 (71.88)
83 (98.81) 1 (1.19)
2 (2.38) 30 (35.71) 0 (0.00) 26 (30.95) 16 (19.05) 3 (3.57) 7 (8.33)
41 (48.81) 5 (5.95) 38 (45.24)
45 (53.57)
57 (67.86)
52.04 ± 61.05 93.54 ± 13.92 1.04 ± 0.15 28.44 ± 5.28 97.79 ± 16.44 3.29 ± 0.90 1347.63 ± 357.09
P
0.876 0.348 0.377
0.525 0.365
1.000
0.105
0.118
0.217
0.522
Patients
Male/female Age Ethnicity
Caucasian Asian Other
Body surface area Previous pregnancy Treatment modality
Inpatients
Outpatients
Primary diagnosis
Acute lymphoblastic leukemia Acute myeloid leukemia Chronic leukemia
Multiple myeloma Non-Hodgkin lymphoma Hodgkin lymphoma
Other Treatment
Autologous stem cell transplantation Allogenic stem cell transplantation Chemotherapy only
Transfusion history Platelets
Red blood cells
Laboratory values prior (< 12 hours) to 1st transfusion
n
n/n
Years, mean ± SD
n (%)
n (%)
n (%)
m2, mean ± SD n/total n of women (%)
n (%) n (%)
n (%) n (%) n (%) n (%) n (%) n (%) n (%)
n (%) n (%) n (%)
n (%)
n (%)
Platelet count
Hemoglobin
International normalized ratio Activated partial thromboplastin time Prothrombin time
Fibrinogen
D-Dimer
109/L, mean
g/L, mean ± SD
mean ± SD
s, mean ± SD %, mean ± SD g/L, mean ± SD ng/mL, mean ± SD
±SD
UVC: ultraviolet C; SD: standard deviation: INR: international normailzed ratio; s: seconds.
for the PP analyses consisted of 146 patients. One UVC arm patient who withdrew his informed consent after the first platelet transfusion but agreed to further documenta- tion of adverse events was included in the ITT and PP populations. The planned safety follow-up could not be carried out in a total of six patients. There were no signif- icant differences in the patient characteristics of the two study groups (Table 1).
Transfusions
In the ITT set, a total of 568 platelet units were trans- fused, 320 to patients in the UVC arm and 248 to those in the control arm. In the PP set, a total of 432 platelet units were transfused, 249 to patients in the UVC arm and 183 to those in the control arm (Tables 2-4). Most of the trans- fusions were platelets administered as single units given prophylactically and were performed with apheresis
platelet units due to higher recruitment rates in study cen- ters that were using apheresis platelets only (Table 2). The mean pre-transfusion platelet count was about 12x109/L and did not differ between the two arms. In the control arm, the majority of transfused platelet units (ITT: 77.8%; PP: 78.1%) were γ-irradiated. Only 4% of platelet transfu- sions in the UVC arm and 5% in the control arm were off- protocol transfusions (Online Supplementary Table S1). Platelet characteristics were similar between study arms.
Platelet transfusion efficacy
All patients in the UVC arm and 96% (81 of 84) of the controls were evaluable for analysis in the ITT population. The mean 1-hour CCI value, the primary outcome, was 12.70% (95% CI: 11.42-13.97) in the UVC group and 15.53% (95% CI: 14.18-16.88) in the control group. The mean difference in 1-hour CCI between the control and
1088
haematologica | 2021; 106(4)