Page 16 - 2021_02-Haematologica-web
P. 16
Editorials
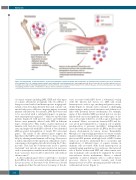
Figure 1. During lifetime, acquired mutations, such as point mutations, structural variants and aneuploidies, in hematopoietic progenitors give rise to accumula- tion of immune the cells carrying somatic variants associated with clonal hematopoiesis. Harboring genetically altered leukocytes is associated with disease vul- nerability and could be linked with dysregulation of normal immune cell functions. Known risk factors for common diseases and clonal hematopoiesis are largely overlapping and their relative contributions to disease risks need further evaluation.
330
of somatic variants (including LOY, CHIP and other types of somatic alterations) in immune cells. In addition to being associated with clonal hematopoiesis in aging indi- viduals, it has been hypothesized that such somatic vari- ants in leukocytes could have a negative impact on normal immune cell functions. For instance, LOY has been found to be associated with a substantial impact on genome- wide transcriptional regulation27-30 while we reported that patients diagnosed with prostate cancer and Alzheimer disease were primarily affected with LOY in different types of leukocytes.29 Our studies of gene expression in immune cells from blood (i.e., different types of leuko- cytes and single cells with LOY collected in vivo) identified LOY-associated dysregulation of nearly 500 autosomal genes.29 The nature of the affected genes support the hypothesis that LOY could contribute to disease vulnera- bility by altering normal immune cell biology. However, it is still not known how the expression of hundreds of auto- somal genes is dysregulated in cells after losing the Y chro- mosome. That said, the Y chromosome in not a ‘genetic wasteland’ and encodes proteins involved in processes such as transcription, translation and chromatin modifica- tions,29 processes that would be affected in cells without chromosome Y.
A direct physiological effect could help to elucidate how LOY in blood is associated with an increased risk of disease in tissues of the entire body. However, it should be emphasized that the risk factors for many of the dis-
eases associated with LOY show a substantial overlap with the known risk factors for LOY and clonal hematopoiesis, such as age, smoking and genetic suscep- tibility (Figure 1). Clearly, such covariance is challenging when trying to make causal inferences and deduction of links with potential disease mechanisms. For example, many of the identified LOY-associated risk loci are also linked with cancer susceptibility and other types of dis- ease, such as type 2 diabetes, as well as age at menopause in women.4 Hence, associations between LOY and dis- ease are in part explained by a ‘common soil’ of genetic predisposition to genomic instability,4 independently associated with an increased risk of LOY in the blood and disease development in various tissues. Remarkably, Ouseph et al.1 report a high prevalence of somatic variants in genes linked with myeloid neoplasia and CHIP in sam- ples from MDS patients with high levels of LOY. Further studies are needed to understand the co-occurrence and implications of different types of somatic variants in immune cells associated with clonal hematopoiesis in normally aging populations. In conclusion, the increased risk of disease in men with LOY and other somatic vari- ants in immune cells can likely be explained by several non-exclusive disease mechanisms. Hence, a reduced capability of altered immune cells to combat disease processes (driven by somatic variants and clonal hematopoiesis) would be operating in parallel with more traditional risk factors (Figure 1).
haematologica | 2021; 106(2)