Page 134 - 2021_02-Haematologica-web
P. 134
S. Jiang et al.
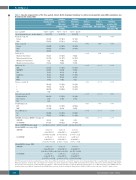
Table 1. Baseline characteristics of the three patient cohorts: Berlin (training), Heidelberg no statins/ursodeoxycholic acid (UDA) (validation), and Heidelberg with statins/UDA.
Berlin cohort (training cohort) (n=446)
01/2013 – 12/2015
54 (18-75)
172 (39) 274 (61)
124 (28) 274 (61) 48 (11)
88 (20) 263 (59) 6 (1) 89 (20)
229 (51) 82 (18) 35 (8) 41 (9) 39 (9) 20 (4)
Peripheral blood
Bone marrow
NA 1(0)
Heidelberg
no statins/ UDA cohort (validation cohort) (n=380)
09/2001 – 01/2010
50 (17-70)
153 (40) 227 (60)
121 (32) 259 (68)
141 (37) 140 (37) 14 (4) 85(22)
101 (27) 53 (14) 39 (10) 96 (25) 75 (20) 16 (4)
131 (34) 65 (17) 184 (48)
351 (92) 29 (8)
291 (77) 89 (23)
193 (51)
187 (49)
32 (8)
348 (92)
(d0 to d+24)
Heidelberg with statins/ UDA cohort (n=359)
01/2010 – 12/2015
56 (19-75)
140 (39) 219 (61)
122 (34) 237 (66)
94 (26) 196 (55) 11 (3) 58 (16)
126 (35) 69 (19) 20 (6) 100 (28) 36 (10) 8 (2)
122 (34) 122 (34) 115 (32)
336 (94) 23 (6)
294 (82) 64 (18)
259 (72)
100 (28)
6 (2)
353 (98)
d+10 (d+1 to d+17)
n=5, rest
of data NA: 1.7 (0.7-3.7; 1.0-2.7) n=347, rest of data NA: 1.0
(0.3-9.1; 0.8-1.7)
n=6 rest
of data NA: 7.4 (4.0-17.1; 5.8-14.8) n=353, rest
of data NA: 7.5
(0.2-195.8; 2.1-14.9)
P
Berlin vs. Heidelberg no statins/UDA
0.67
P
Berlin vs. Heidelberg with statins/UDA
0.942
P
Heidelberg no statins/UDA vs. Heidelberg
with statins/UDA
0.764
Date of alloSCT
Age at transplant in years - median (range) Recipient sex (n, %)
Female
Male
Donor sex (n, %)
Female Male NA
Donor (n, %)
Matched related donor Matched unrelated donor Mismatched related donor Mismatched unrelated donor
Disease (n, %)
AML MDS/MPN ALL Lymphoma MM
Other
Disease score (n, %) 0
1
2
NA 9(2)
Stem-cell source (n, %)
0.877 0.437 0.584
142 (32) 47 (11) 248 (56)
<0.001
<0.001
0.012
<0.001
0.999
<0.001
0.472
0,034
<0.001
<0.001
<0.001
0.055
<0.001
<0.001
<0.001
<0.001
<0.001
0.566
0.069
<0.001
<0.001
443 (99) 2 (1)
Conditioning (n, %) RIC
MAC
Use of ATG (n, %)
Yes
No
SOS/VOD development (EBMT criteria)(n, %)
341 (76) 105 (24)
399 (89)
47 (11)
SOS/VOD
No SOS/VOD
Onset of SOS/VOD (median, range) Median CIBMTR score (range, IQR)
SOS/VOD
No SOS/VOD
Median EASIX-d0 (range, IQR)
SOS/VOD
No SOS/VOD
43 (10)
401 (90)
d+9 (d+3 to d+30)
n=32, rest
of data NA: 1.51 (0.56-4.48; 0.82-2.37) n=338, rest
of data NA: 1.01 (0.32-9.72; 0.76-1.80)
n=41, rest
of data NA: 40.26:
d+8
n=29, rest of data NA: 1.9 (0.3-8.7; 1.4-3.1) n=333, rest of data NA: 1.2
(0.4-20.6; 0.9-2.1)
n=32, rest of data NA: 8.6
(5.23-865.06; 14.72-80.38) (0.2-41.0; 3.4-15.4)
n=376, rest
of data NA: 16.06 (0.38-575; 6.00-36.54)
n=348, rest of data NA: 2.3
(0.2-99.2; 0.9-7.5)
SOS/VOD: sinusoidal obstruction syndrome/veno-occlusive disease; UDA: ursodeoxycholic acid; alloSCT: allogeneic stem cell transplantation; NA: not available; AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; ALL: acute lymphocytic leukemia; CLL: chronic lymphocytic leukemia; MPN: myeloproliferative neoplasms; CML: chronic myeloid leukemia; MM: multiple myeloma; RIC: reduced intensity conditioning; MAC: myeloablative conditioning; ATG: anti-thymocyte globulin; KPS: Karnofsky Performance Score; IQR: interquartile range; CIBMTR: Center for International Blood and Marrow Transplant Research; EBMT: European Group for Blood and Marrow Transplantation; EASIX-d0: EASIX assessed at the day of alloSCT; n: number; d: day.
448
haematologica | 2021; 106(2)