Page 13 - 2021_02-Haematologica-web
P. 13
Editorials
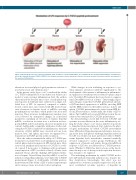
Figure 1. Physiological responses to lipopolysaccharide (LPS), an inducer of acute inflammation, are modulated in mice by pretreatment with the C-terminal por- tion of the endocrine hormone FGF23 (C-FGF23). The attenuated responses involve parameters that are relevant to systemic iron balance and the development of the anemia of inflammation.
alterations in several physiological parameters relevant to iron homeostasis and erythropoiesis.13
In the present study, Agoro et al.2 considered the ability of C-FGF23 administration to modulate iron balance in a model of acute systemic inflammation. First, the authors characterized the detailed time course of early physiolog- ical responses in wild-type mice subjected to a single, sub- lethal dose of LPS. As expected, compared to vehicle- treated control mice, mice treated with LPS showed tran- sient elevations in hepatic levels of mRNAs encoding proinflammatory cytokines, which peaked one hour after injection. These increases in inflammatory markers were soon followed by anticipated changes in iron-related parameters, including an elevation of hepatic hepcidin mRNA, a reduction in serum iron, a reduction in ferro- portin mRNA in liver and spleen, and a rise in splenic iron content. Additionally, compatible with prior studies indi- cating that FGF23 is regulated by inflammation, transient elevations of Fgf23 mRNA were observed in bone, bone marrow, liver, and spleen, and were accompanied by a transient rise in FGF23 protein in the circulation.
Next, the authors examined the ability of C-FGF23 pre- treatment to modulate these acute inflammatory and iron- related physiological responses in LPS-treated mice. Mice received an intraperitoneal injection of C-FGF23 (or vehi- cle control) 8 hours prior to challenge with an intraperi- toneal injection of LPS (or vehicle control); all mice were analyzed 4 hours after the challenge. LPS-mediated induc- tion of hepatic Tnf and Hamp (hepcidin) mRNA, as well as induction of hyperhepcidinemia, was blunted by C-FGF23 pretreatment (Figure 1). Additionally, C-FGF23 pretreat- ment attenuated the ability of LPS to induce hypoferremia and to increase tissue iron levels in liver and spleen.
While changes in iron trafficking in response to sys- temic immune activation contribute significantly to the development of the anemia of inflammation, inflammato- ry suppression of erythropoietic activity also plays a major role.1 Notably, LPS-treatment has been shown to suppress renal Epo mRNA levels in rodents.14 Interestingly, Agoro and colleagues found that C-FGF23 pretreatment alleviat- ed LPS-mediated suppression of mRNAs encoding EPO and the EPO receptor in the kidney and also in liver and spleen. C-FGF23 pretreatment also raised serum EPO lev- els in LPS-treated mice. Additionally, the ability of LPS to induce Fgf23 mRNA in bone and FGF23 protein in the cir- culation was attenuated by C-FGF23 pretreatment.
By demonstrating a novel link between C-FGF23 and systemic iron modulation in the acute inflammatory set- ting, the study of Agoro et al.2 raises a number of questions to stimulate future investigation. First, what are the phys- iological mechanisms by which C-FGF23 pretreatment alters LPS-induced hypoferremia and attenuates iron stor- age in the liver and spleen? Although hepcidin induction by LPS was blunted in mice pretreated with C-FGF23, serum hepcidin levels nevertheless remained markedly elevated compared to control groups. Furthermore, the suppression of hepatic and splenic ferroportin mRNA in response to LPS was not alleviated by C-FGF23 pretreat- ment. Whether C-FGF23 has direct actions on the duode- num, the major site of dietary iron absorption in the intes- tine, remains to be explored.
The underlying mechanism by which C-FGF23 modu- lates LPS-mediated hepcidin induction also requires fur- ther clarification. Interestingly, this effect was not explained by a reduction in hepatic expression of IL-6, the most established cytokine regulator of hepcidin in
haematologica | 2021; 106(2)
327