Page 118 - 2021_02-Haematologica-web
P. 118
M. Ichii et al.
AB
CD
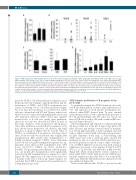
Figure 7. STAP-2 expression under hematologic stress. (A-C) The indicated subsets (pro-B; B220+ CD43+ CD19+ IgM–, pre-B; B220+ CD43– CD19+ IgM–, Lin(–); lineage marker negative, LSK; Lineage– Sca1+ cKithigh, common lymphoid progenitor [CLP]; Lin– Sca1+ cKitlow Flk2high IL7Rα+) of hematopoietic cells derived from wild-type (WT) mice three days after LPS administration (A) (n=6 in each), or 12 hours (B) (control, n=6; with irradiation, n=13) or one month (C) (control, n=3; with irradiation, n=5) after sub-lethal irradiation (3.5 Gy) were analyzed for Stap-2 expression by real-time polymerase chain reaction (RT-PCR). Stap-2 transcript levels were normalized to the median of control samples without treatment. (D) Indicated cytokines (IFN-γ, 0.1 ng/mL; IL-6, 50 ng/mL; IL-1β, 5.0 ng/mL; TNFα, 50 ng/mL; LPS, 5.0 mg/mL) were added to B-progenitor cultures. Cells were collected after 24-hour stimulation, and RT-PCR was performed. Results are expressed as fold change relative to the controls, and are representative of results obtained in three independent experiments. Results are shown as mean ± standard deviation. Statistical significances relative to controls without stimulation were determined by unpaired two-tailed Mann-Whitney tests: *P<0.05.
mised by STAP-2. Sub-lethal irradiation eliminates most hematopoietic cells, leading to rapid proliferation and dif- ferentiation of HSPC, and STAP-2 requirement was assessed following 3.5 Gy sub-lethal irradiation (Figure 3C). Four days post irradiation, loss of myeloid, T, and B cells was observed in both WT and Tg mice, whose total white blood cell counts were indistinguishable before and after irradiation. However, while control mice regained normal levels of B cells two weeks after irradiation, almost no recovery was observed in STAP-2 Tg mice at that stage. Analyses of the proliferation and apoptosis status after irradiation revealed that B progenitors in Tg mice showed more progressive cell division, and rapid induction of apoptosis (Figure 3D). To test the effect of STAP-2 on undamaged B progenitors following direct stress, B-cell recovery was monitored after congenic BM transplantation. One month after transplantation to lethally irradiated recipients, CD45.2+ donor chimerism in blood B cells of Tg LSK-transplanted recipients was sig- nificantly lower than in WT transplanted mice (WT donor, 63.3±7.5%; STAP-2 Tg donor, 23.0±2.8%; P<0.001). The percentage and cellularity of BM B progen- itors one month after transplantation were significantly reduced in Tg LSK transplanted mice compared to control (Figure 3E). The suppression of B-cell recovery lasted for 4 months at the end of follow-up. Taken together, we concluded that STAP-2 governs B-cell reconstitution fol- lowing hematologic stress.
STAP-2 impairs proliferation of B progenitor at the pre-B stage
To gain further insight into STAP-2 functions, we evalu- ated B-cell differentiation of Stap-2 gene targeted mice in vitro. The capacity for B-cell lineage development was reduced, when HSPC derived from Tg were cultured under stromal-cell free, B-cell conditions in the presence of IL-7, FL and SCF (Figure 4A). The same was true in cul- tures of LSK cells as well as CLP with or without OP9 stro- mal cells (Figure 4B and C).
After rearrangement and expression of immunoglobulin (Ig) heavy chain loci at the pro-B stage, large pre-B cells enter cell-cycle and undergo several rounds of prolifera- tion. The Ig light chain gene is then rearranged, and cells begin to express a functional B-cell receptor, progressing to an IgM+ immature B-cell stage. Colony-forming unit (CFU) assays were used to study the effect on pre-B-cell proliferation, and we found that STAP-2 deficiency pro- moted the generation of pre-B colonies, while overexpres- sion of STAP-2 inhibited colony recovery with statistical significance (recovered colony number; 90.0±9.5 in WT, 40.7±3.1 in Tg; P<0.001, and 200.0±5.3 in KO; P<0.001) (Figure 4D). Under stromal-cell free culture conditions of Mac1– B220+ CD19+ CD43+ IgM– pro-B cells, STAP-2 regu- lated the expansion in the same way as was found in the CFU assay, while the differentiation into immature B cells was unaffected (Figure 4E).
These results indicate that STAP-2 is involved in the
432
haematologica | 2021; 106(2)