Page 53 - 2020_11-Haematologica-web
P. 53
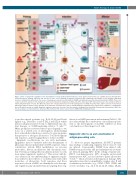
Figure 1. Role of epigenetic regulators in the development of acute graft-versus-host-disease. Acute graft-versus-host disease (aGvHD) develops through three sequential phases: priming, induction and effector. In some cases, following prophylactic treatment and conditioning, the integrity of the intestinal epithelium becomes compromised and leads to the release of damage-associated molecular patterns (DAMP) and pathogen-associated molecular patterns (PAMP). These mol- ecules result in the activation of hematopoietic and non-hematopoietic antigen-presenting cells (APC). Subsequent APC interactions lead to the activation, differen- tiation and proliferation of T cells. The different subsets of T cells play numerous roles in the pathogenesis of aGvHD. Th1, Th2, Th17, and the cytotoxic T cells interact with target organs to promote tissue damage. In the intestines, intestinal stem cells are notably damaged, impairing tissue regeneration capabilities, contributing to the feed-forward cascade of aGvHD. Epigenetic regulators play a role in each of the three phases allowing for the possibility of therapeutic interventions. HDAC: his- tone deacetylase; IL: interleukin; DLL: delta-like; IFN: interferon; TNF: tumor necrosis factor; DNMT: DNA methyltransferases
Basic biology of acute GvHD
to produce special cytokines (e.g., IL-12, IL-23) and Notch ligands (e.g., Delta-like 1 and 4; DLL1 and DLL4) which instruct antigen-activated T cells to differentiate into dis- tinct lineages of GvHD-mediating effector T cells.5,12-15 Other groups have reviewed these topics elegantly, so we focus on a related area of investigation: understanding how extracellular stimuli are converted to gene programs that promote or abrogate alloreactive T-cell development and responses, and leveraging them to reduce aGvHD.
Epigenetic modifications are one such mechanism. Epigenetics refers to heritable molecular determinants of phenotype that are independent of DNA sequence. Major contributors include DNA methylation on cytosine nucleotides, histone modification and chromatin struc- ture. Proteins governing these modifications have loosely been termed epigenetic regulators.16 This review will dis- cuss advances in our understanding of epigenetic regula- tion, either by direct effects or via interactions with other molecules, of alloreactive T-cell responses and these responses’ roles in aGvHD; we identify the roles that spe- cific regulators play and interventions targeting these reg-
ulators for aGvHD prevention and treatment (Table 1). We also acknowledge the contributions of non-hematopoietic cells to the development of aGvHD, whether via their own function or their impact on T cells.
Epigenetic effects on and sensitization of antigen-presenting cells
To allow for proper engraftment, allo-HSCT patients may undergo conditioning regimens before donor T cells are infused. Consequently, DAMP from injured cells, PAMP from gut bacteria and pro-inflammatory cytokines are released, priming APC.1 In the setting of murine allo- HSCT, non-hematopoietic APC, alongside professional hematopoietic APC, are also known to prime alloreactive T cells.2,6,17 Upon activation following tissue damage, APC upregulate major histocompatibility complex class II and costimulatory molecules (e.g., CD40, CD80, CD86) and secrete cytokines (e.g., IL-4, IL-12, IL-23, DLL1, DLL4),
haematologica | 2020; 105(11)
2541