Page 159 - 2020_09-Haematologica-web
P. 159
Mutated factor X to correct hemophilias
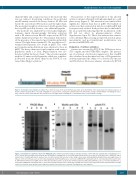
chain (20 kDa) and a minor fraction of the molecule that was not reduced. In reducing conditions, the polyclonal anti-FX mainly revealed the heavy chain at 50 kDa and barely the non-reduced FX fraction and the light chain. The molecular weight of actiten was 3-4 kDa greater than that of pdFX, probably because of its additional peptide.
The molecule was analyzed by reverse phase high-per- formance liquid chromatography following activation with a FX activator from Russell’s viper venom (RVV-X) (Online Supplementary Figure S1). This analysis demonstrat- ed the presence of the expected post-translational modifi- cations. The light chain contained 11 Gla, in addition to C- terminal heterogeneity, also found in pdFX. The other post-translational modifications were identical to those in pdFX. The heavy chain was found complete with a minor population under a β form. O-glycosylation was also detected within the heavy chain.19 The activation peptide with the 10 amino-acid polypeptide added was complete- ly liberated from the heavy chain by the RVV-X. It con- tained three O-glycosylations.19
The presence of the expected number of Gla allowed actiten to interact efficiently with phospholipids in a solid phase assay (Figure 3). The interaction was similar albeit slightly less efficient than that of pdFX. The half-life of actiten was then evaluated in rabbits in which pdFX had been previously studied. Both products had a similar half- life (at around 6 h) indicating that the modification of the FX did not affect its pharmacokinetics (Online Supplementary Figure S2). These data indicate that a mutat- ed recombinant FX possessing an expected structure, phar- macokinetics and post-translational modifications was produced in HEK293F cells.
Evaluation of actiten activation
Actiten was activated by RVV-X, the FVIIa/tissue factor (TF) complex and the FVIIIa/FIXa complex. The percent- age of activation of actiten in comparison to that of pdFX was calculated from the initial velocity of FXa generation. Actiten maintained the ability to be activated by the nat- ural FX effectors. However, whereas activation by RVV-X
Figure 1. Schematic representation of actiten. The scheme focuses on the factor X (FX) heavy chain: at the N-terminus of the heavy chain, the natural sequence of the FX activation peptide (AP) 1→52 ends at Arg234; in red a 10-amino acid peptide added between the AP and the catalytic domain (IVGGQ--) modifying the speci- ficity of FX. The yellow arrow indicates the activation site. The light chain containing the g-carboxylation sites is represented in orange.
ABC
Figure 2. Visualization of purified actiten from HEK293F cells. Actiten was purified using an anti-Gla aptamer column and was separated by electrophoresis on a 4-12% sodium dodecylsulfate polyacrylamide gel. (A) PAGE Blue staining. Lane 1, molecular weight (values in kDa are on the left of the gel); lane 2, HEK293F super- natant; lane 3, flow-through; lane 4, washes; lane 5, purified actiten. (B) Detection of Gla using a monoclonal antibody (MoAb anti-Gla). Molecular weight values in kDa are on the left of the gel. Lane 1, non-reduced plasma-derived FX (pdFX); lane 2, non-reduced HEK293F supernatant; lane 3, non-reduced purified actiten; lane 4, dithiothreitol (DTT)-reduced pdFX; lane 5, DTT-reduced purified actiten. (C) Detection of FX by polyclonal antibodies (pAnti-FX). Lane 1, non-reduced pdFX; lane 2, non-reduced HEK293F supernatant; lane 3, non-reduced purified actiten; lane 4, DTT-reduced pdFX; lane 5, DTT-reduced purified actiten. Separated lanes from (B) and (C) are from the same gel but some bands not related to this article were removed. The signals were not adjusted.
haematologica | 2020; 105(9)
2337