Page 85 - Haematologica May 2020
P. 85
Extensive mixed chimerism analysis in sickle cell disease post HSCT
P = 0.313
P = 0.016 P = 0.03
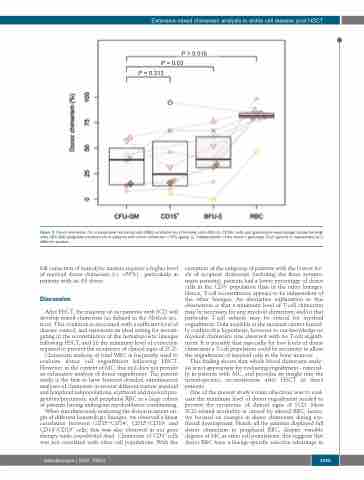
Figure 3. Donor chimerism (%) in peripheral red blood cells (RBC), erythroid burst forming units (BFU-E), CD15+ cells and granulocyte-macrophage colony-forming- units (CFU-GM) progenitors/precursors in patients with donor chimerism <70% (group 1), independently of the donor’s genotype. Each patient is represented by a different symbol.
full correction of hemolytic anemia requires a higher level of myeloid donor chimerism (i.e. >50%) - particularly in patients with an AS donor.
Discussion
After HSCT, the majority of our patients with SCD will develop mixed chimerism (as defined in the Methods sec- tion). This condition is associated with a sufficient level of disease control, and represents an ideal setting for investi- gating (i) the reconstitution of the hematopoietic lineages following HSCT, and (ii) the minimum level of correction required to prevent the recurrence of clinical signs of SCD.
Chimerism analysis of total WBC is frequently used to evaluate donor cell engraftment following HSCT. However, in the context of MC, this tool does not provide an exhaustive analysis of donor engraftment. The present study is the first to have featured detailed, simultaneous analyses of chimerism in several different mature myeloid and lymphoid subpopulations, erythroid and myeloid pro- genitors/precursors, and peripheral RBC in a large cohort of patients having undergone myeloablative conditioning.
When simultaneously analyzing the donor/recipient ori- gin of different hematologic lineages, we observed a linear correlation between CD15+/CD14+, CD15+/CD19+ and CD14+/CD19+ cells; this was also observed in our gene therapy trials (unpublished data). Chimerism of CD3+ cells was not correlated with other cell populations. With the
exception of the subgroup of patients with the lowest lev- els of recipient chimerism (including the three sympto- matic patients), patients had a lower percentage of donor cells in the CD3+ population than in the other lineages. Hence, T-cell reconstitution appears to be independent of the other lineages. An alternative explanation to this observation is that a minimum level of T-cell chimerism may be necessary for any myeloid chimerism, and/or that particular T-cell subsets may be critical for myeloid engraftment. Data available at the moment cannot formal- ly confirm this hypothesis, however to our knowledge no myeloid chimerism was observed with no T-cell engraft- ment. It is possible that especially for low levels of donor chimerism a T-cell population could be necessary to allow the engraftment of myeloid cells in the bone marrow.
This finding shows that whole blood chimerism analy- sis is not appropriate for evaluating engraftment - especial- ly in patients with MC, and provides an insight into the hematopoietic reconstitution after HSCT in these patients.
One of the present study’s main objectives was to eval- uate the minimum level of donor engraftment needed to prevent the recurrence of clinical signs of SCD. Most SCD-related morbidity is caused by altered RBC; hence, we focused on changes in donor chimerism during ery- throid development. Nearly all the patients displayed full donor chimerism in peripheral RBC, despite variable degrees of MC in other cell populations; this suggests that donor RBC have a lineage-specific selective advantage in
haematologica | 2020; 105(5)
1245