Page 30 - Haematologica May 2020
P. 30
N. Curto-Garcia et al.
patients.9-11 Up to 20% with ET and up to 15% of patients with PMF lack detectable mutations in these three genes, as assessed by conventional assays; such patients are termed ‘triple negative’.12-14 Lastly, comprehensive genom- ic analyses have revealed the presence of additional muta- tions that can appear before, simultaneously, or following the so-called ‘driver mutations’ (JAK2, CALR and MPL) in PMF and can affect a wide-array of key genes, such as those involved in epigenetic regulation (TET2, ASXL1, EZH2), splicing (SRSF2, U2AF1), and cellular signaling (SH2B3, PIAS3), some of which also affect prognosis.15
Multiple factors contribute to the dynamic complexity of the bone marrow niche in MPN, such as the inherent increase in pro-inflammatory cytokines, skewed adaptive and innate immune responses, and ‘cross-talk’ between the normal and mutated-HSC, endosteal and vascular niches and extracellular matrix. In this review, we will summarize current knowledge concerning bone marrow niche composition in health and how it differs in MPN. Likewise, as we gain further understanding of these dynamics, we will explore what potential there is for ther- apeutic intervention specifically targeting the niche to pro- vide clinical benefit.
Overview of the bone marrow niche in health
It is evident that much remains to be elucidated concern- ing the dynamic BM microenvironment, both in normal physiological and disease states. Traditionally, the niche is conceived as being divided into individual compartments
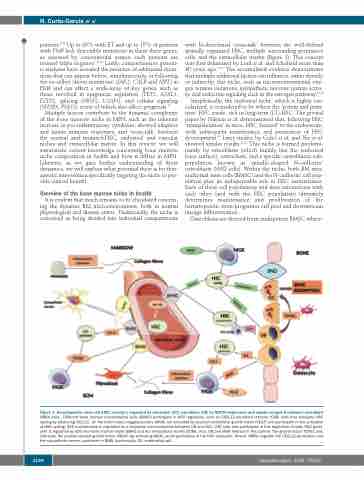
with bi-directional ‘cross-talk’ between the well-defined spatially organized HSC, multiple surrounding permissive cells, and the extracellular matrix (Figure 1). This concept was first delineated by Lord et al. and Schofield more than 40 years ago.16,17 The accumulated evidence demonstrates that multiple additional factors can influence, either directly or indirectly, this niche, such as microenvironmental oxy- gen tension variations, sympathetic nervous system activi- ty, and endocrine signaling such as the estrogen pathway.18,19
Simplistically, the endosteal niche, which is highly vas- cularized, is considered to be where the ‘potent and prim- itive’ HSC reside, rich in long-term (LT)-HSC. The pivotal paper by Nilsson et al. demonstrated that, following HSC ‘transplantation’ in mice, HSC ‘homed’ to the endosteum, with subsequent maintenance and promotion of HSC development.20 Later studies by Celso et al. and Xie et al. showed similar results.21,22 This niche is formed predomi- nantly by osteoblasts (which mainly line the endosteal bone surface), osteoclasts, and a specific osteoblastic sub- population known as spindle-shaped N-cadherin+ osteoblasts (SNO cells). Within the niche, both BM mes- enchymal stem cells (BMSC) and the N-cadherin+ cell pop- ulation play an indispensable role in HSC maintenance. Each of these cell populations and their interactions with each other (and with the HSC population) ultimately determines maintenance and proliferation of the hematopoietic stem/progenitor cell pool and downstream lineage differentiation.
Osteoblasts are derived from multipotent BMSC where-
Figure 1. Hematopoietic stem cell (HSC) cycling is regulated by osteoclast (OC), osteoblast (OB) by NOTCH expression and spindle-shaped N-cadherin+ osteoblast (SNO) cells. Different bone marrow mesenchymal cells (BMSC) participate in HSC regulation, such as CXCL12-abundant reticular (CAR) cells that stimulate HSC cycling by producing CXCL12. On the other hand, megakaryocytes (MGK) are activated by vascular endothelial growth factor (VEGF) and participate in the activation of HSC cycling. HSC maintenance is regulated by a reciprocal communication between OB and HSC. CAR cells also participate in this regulation. Finally, HSC quies- cent is regulated by both the bone marrow niche (BMN) and the extracellular matrix (ECM), thus, OB and MGK interact in this control. The growth factor TGFb1 and, indirectly, the platelet-derived growth factor (PDGF) (by activating MGK) would participate in the HSC quiescent. Nestin+ BMSC regulate the CXCL12 production and the sympathetic nerves contribute to BMN functionality. EC: endothelial cell.
1190
haematologica | 2020; 105(5)