Page 14 - 2019_03-Haematologica-web
P. 14
Editorials
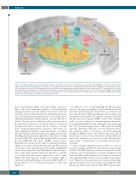
Figure 1. The mitochondrial associated membrane. Hundreds of proteins operate at the mitochodrial-associated membrane (MAM) but the location of proteins dis- cussed in this editorial are illustrated here. A major route of communication between the endoplasmic reticulum (ER) and mitochondria occurs through the release of ER calcium via the inositol 1,4,5-trisphosphate receptor (IP3R), the volatage-gated anion channel (VDAC) and the chaperone GRP75. Calcium gains access to the matrix through the mitochondrial Ca2+ uniporter (MCU) leading to membrane depolarization and cytochrome C release as part of the apoptotic pathway. This exchange of calcium is triggered by the interaction of fission protein 1 (FIS1), B-cell receptor-associated protein 31 (BAP31), and caspase 8 within the MAM upon apoptotic stimuli, such as combination treatment with etoposide and PS89.
420
tion, mitochondrial shape and, importantly, apoptosis. One of the most important regulators of mitochondrial energy production and cell death is the release of ER cal- cium into the mitochondria thereby facilitating the open- ing of the permeability transition pore at the inner mito- chondrial membrane, depolarization, and cytochrome C release. This process is orchestrated by a vast network of proteins that could serve as potential targets as evidenced by their alteration in cancer cells as a means of escape from chemotherapy-induced apoptosis that relies on Ca2+ signaling.5 A number of proteins appear to stabilize ER-mitochondria contact thereby prolonging calcium flux including mitofusin-2, phosphofurin acidic cluster sorting protein 2 (PACS-2), and double-stranded RNA-activated protein (PKR)-like ER kinase (PERK) among others. Modulation of such contacts and crosstalk may facilitate apoptosis in cancer cells particularly since the Bcl-2 family of proteins reside and interact at the MAM, highlighted by the increasing interest in BH3-mimetics that alter pro- teins of this interconnected network. Likewise, a number of oncoproteins and tumor suppressors such as p53, PTEN and AKT function locally. For example, p53 is enriched on the MAM where it interacts with the ER Ca2+ pump SERCA to boost ER-mitochondrial Ca2+ flux and apoptosis.6
In addition to Ca2+ storage/signaling, the ER also plays a major role in protein synthesis, post-translational mod- ifications and folding. ER homeostasis is a delicate bal- ance and when the folding machinery can no longer keep up with protein synthesis an adaptive response called the unfolded protein response (UPR) occurs. This response seeks to restore balance by attenuating protein transla- tion, upregulating ER protein degradation and increasing the level of chaperone proteins to inflate protein folding capacity.7 When the UPR fails to restore ER proteostasis, the pathway shifts to promote cell death primarily though the PERK branch of the UPR. In this way, the ER functions as a sensor of protein stress and perturbations lead to the induction of a variety of survival/death path- ways, many of which rely on crosstalk with the mito- chondria.
Correct folding of many proteins (e.g. 80% of secretory proteins) requires disulfide (S-S) bonds between cysteine residues. The PDI family of proteins is responsible for the formation and rearrangement of protein disulfide bonds and these ER-resident enzymes also function as chaper- ones independently of their role in disulfide bond forma- tion.8 Therefore, these proteins are essential in maintain- ing protein homeostasis at baseline and during the UPR. Numerous studies have indicated that PDIA1 (gene name,
haematologica | 2019; 104(2)