Page 17 - Haematologica-April 2018
P. 17
Editorials
CD83 in Hodgkin lymphoma
Ralf Küppers
Institute of Cell Biology (Cancer Research), University of Duisburg-Essen, Medical Faculty, Germany
E-mail: ralf.kueppers@uk-essen.de doi:10.3324/haematol.2018.188870
The development of combined chemotherapy with or without radiotherapy for classical Hodgkin lym- phoma (HL) can be considered as a major success story in onocology. With current treatment protocols, a long-term cure is obtained in about 80-90% of patients.1 However, these therapies come with considerable toxicity and a risk for the development of secondary cancers, which is particularly problematic not only for non-fit eld- erly patients, but also for young adult HL patients. Hence, there is currently much concentrated effort being put into the development of a more targeted and less toxic therapy. One very promising approach is the use of a toxin-coupled anti-CD30 antibody, brentuximab vedotin, which directly targets the Hodgkin and Reed-Sternberg (HRS) tumor cells in HL, as they consistently express high levels of CD30.1 A second targeted therapy with exciting results from clinical studies involves antibodies against programmed cell death- 1 (PD-1) or programmed cell death ligand 1 (PD-L1).1 PD- L1 is expressed by HRS cells and inhibits PD-1-expressing activated T cells as a means of immune evasion.2 In this issue of Haematologica, Li and colleagues focus on CD83 as a further potentially attractive candidate, both as a bio- marker and target for therapy.3
CD83 is a membrane glycoprotein belonging to the immunoglobulin superfamily. It is frequently used as a general marker for dendritic cells, but it is also expressed by some other cell types, including a fraction of B cells and T cells.4 CD83 is also released from cells, and the sol-
uble form (sCD83) is even detectable at a low concentra- tion in the serum of healthy individuals. This release seems to be predominantly mediated by proteolytic cleavage from membrane-anchored CD83, but may also involve differential splicing to produce a secreted form. Until recently there was no indication for the CD83 lig- and(s).5 However, a number of studies have since revealed numerous immunosuppressive functions of sCD83.4-6
The expression of CD83 by HRS cells was already described more than 20 years ago by Hart and colleagues.7 A more recent study confirmed the frequent expression of CD83 by HRS cells, and showed that this can serve as a valuable marker to distinguish classical HL from anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma, which can be a difficult differential diagnosis.8 As CD83 was initially considered to be a dendritic cell marker, its expression by HRS cells was originally interpreted as a hint for a dendritic cell ori- gin of HRS cells,7 but we now know that CD83 is also specifically expressed by centrocytes, the non-proliferat- ing subset of germinal center B cells.9 Hence, although HRS cells, which are derived from germinal center B cells,10 have largely lost their B cell typical gene expression pattern,11,12 expression of CD83 by HRS cells in the major- ity of cases of HL may reflect their germinal center B-cell origin. The retained expression of this marker by HRS cells may indicate that it is of selective advantage for HRS cells to keep it expressed and not to downregulate it;
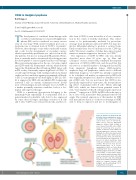
Figure 1. Features of CD83 in HL and potential clinical applications involv- ing CD83. CD83 is expressed on HRS cells in most cases of classical HL, which can be used for differential diagnosis. Soluble CD83 (sCD83) is also released from HRS cells. sCD83 levels in serum may serve as a bio- marker for disease load. sCD83 has immunosuppressive functions when binding to its still poorly characterized ligands on target cells. CD83 can also be transfered to other cells in the HL microenvironment by trogocytosis, a process in which membrane frag- ments are transfered from one cell to another. This process may also be immunosuppressive, as it causes upregulation of PD-1 on T cells, likely rendering them more responsive to inhibiting signals from PD-L1 on HRS cells. Finally, a toxin-coupled mono- clonal antibody against human CD83 has been developed, which efficiently kills HL cell line cells. This antibody needs to be further tested for its suit- ability for targeted therapy. HRS: Hodgkin and Reed-Sternberg; PD-1: programmed cell death-1.
haematologica | 2018; 103(4)
561