Page 138 - 2020_08-Haematologica-web
P. 138
A.K. Abdel-Aziz et al.
lenged for long periods.23 After prolonged exposure of responsive AML (KASUMI-1/P) to DDP38003, secondarily resistant AML cells (KASUMI-1/R) started to grow in the presence of LSD1i. Such KASUMI-1/R cells were cross resistant to another LSD1i. Of note, mTOR activation was observed not only in AML cells intrinsically resistant to LSD1 inhibition, but also as a mechanism of acquired resistance to LSD1 inhibition in primarily sensitive AML cells. Analogously, imatinib triggered mTOR activation in a chronic phase chronic myelogenous leukemia (CML) patient which critically mediated CML survival during the early phase of acquired imatinib resistance before the acquisition of a kinase mutation.22 Although we have not analyzed the eventual genetic alterations in KASUMI-1/R cells, the observation that acquired resistance could be reverted by mTOR inhibition suggests that an adaptive rather than a genetic mechanism is involved in mediating mTOR activation and resistance to LSD1 inhibition. Nonetheless, this shall be systemically investigated in our
future studies on a larger subset of secondarily resistant AML.
Delving deeper, we have investigated how LSD1 differ- entially modulates mTOR in resistant versus sensitive AML cells. Intriguingly, we have observed mTORC1 acti- vation in experimental conditions where AMPK - which in many cases acts as a mTOR inhibitor29 - was activated and thereby excluding its involvement. In line with AMPK stimulation, we found that LSD1i increases the phospho- rylation and hence inactivation of the down-stream target of AMPK, ACC which is the rate-limiting enzyme of fatty acid synthesis. In line with our data, LSD1 knockdown has been shown to reduce the triglyceride levels through modulating sterol regulatory element binding protein (SREBP1)-mediated activation of lipogenic gene transcrip- tion.43 mTOR also promotes de novo lipogenesis via activat- ing SREBP1 and phosphorylating serine/arginine protein kinases, thereby promoting the splicing of lipogenic pre- mRNA.44 Our data highlighting the modulatory effects of
ABC
DE
F
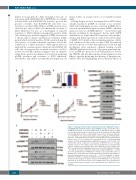
Figure 6. Targeting mTOR sensitizes primary patient-derived MLL-AF9 express- ing (AML-IEO20) leukemia blasts to LSD1 inhibition in vitro. (A) Growth curves of AML-IEO20 leukemic cells treated with vehicle or DDP38003 (0.1 or 0.5 mM) for the indicated time points of treatment (n=3). (B) Assessment of CD11b mRNA levels in AML-IEO20 leukemic cells following 72 hours (h) of treatment with vehi- cle or DDP38003 (0.1 and 0.5 mM) using real-time quantitative PCR (RT-qPCR). Data were statistically analyzed using one way ANOVA followed by Bonferrroni post hoc test.*: P<0.05 compared to vehicle-treated cells. (C) Immunoblotting analysis of mTOR signaling pathway in AML-IEO20 leukemic cells treated for 72 h with either vehicle or different concentrations of DDP38003 (0.1 or 0.5 mM). Vinculin served as the loading control. The presented blots are derived from replicate samples run on parallel gels and controlled for even loading. (D-E) Effect of targeting mTOR signaling using an allosteric mTOR inhibitor (rapamycin – 10 nM) (D) or an ATP competitive mTOR kinase inhibitor (AZD8055 – 10 nM) (E) on the growth kinetics of AML-IEO20 leukemic cells treated with vehicle or DDP38003 (0.5 mM). Data were statistically analyzed using two way ANOVA fol- lowed by Bonferrroni post hoc test (n=3). a,b,c: P<0.05 compared to vehicle, DDP38003/shLSD1 or rapamycin/AZD8055 alone treated groups respectively. (F) Western blot analysis of lysates obtained from AML-IEO20 cells treated as indicated for 72 h. Vinculin served as the loading control. The presented blots are derived from replicate samples run on parallel gels and controlled for even loading.
2114
haematologica | 2020; 105(8)