Page 11 - 2020_08-Haematologica-web
P. 11
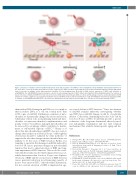
Figure 1. Response of human cord blood (CB) hematopoietic stem and progenitor cells (HSPC) to low- and high-dose X-ray irradiation demonstrated by Henri et al.10 A low dose of 0.02 Gy (20 mGy) X-rays induces reactive oxygen species (ROS) elevation coupled with decrease in mitochondrial membrane potential (ΔΨ), which leads to increase in oxidative stress represented by formation of 8-oxo-deoxyguanosine (8-oxo-dG) in DNA, nuclear expression of NRF2, and activation of p38/MAPK14. The p38/MAPK14 activation mediates a decline in self-renewing capacity of HSPC without affecting their differentiating potential. The low-dose X- rays do not induce γ-H2AX and 53BP1 foci that represents nuclear DNA double strand breaks (DSB), or canonical DNA damage response via phosphorylation of ATM and p53. In contrast, a high dose of 2.5 Gy X-ray irradiation causes both ROS elevation and nuclear DSB. As a result, ROS inhibition either by N-acetylcysteine (NAC) or catalase, or p38 inhibition by SB203580, can reverse the detrimental effect by low dose, but not high dose, of X-rays on self-renewal capacity of HSPC.
Editorials
than nuclear DNA. Damage in mtDNA is not so simple as that in nuclear DNA, as a cell can contain more than 1,000 copies of mtDNA. Furthermore, numbers of mito- chondria are dynamically changed by fusion and fission, which play critical roles in maintaining functional mito- chondria via inter-mitochondrial complementation and quality control.17 In addition, damaged mitochondria can be removed by autophagy, which contributes to mainte- nance of self-renewal capacity of HSC.18,19 Henry et al. show that mitochondrial mass in HSPC does not seem to change after irradiation of 20 mGy X-rays.10 Although this observation should be validated by other methods,20 it supports the idea that changes in mitochondrial mass are unlikely to be the cause of ROS elevation. Rather, it is tempting to speculate that damage in mtDNA induced by low-dose IR causes persistent changes in mitochondrial function that lead to initial elevation of ROS and long- term impairment of HSC function. This would be consis- tent with the results reported by Rodrigues-Moreira et al., which demonstrate that low-dose X-rays cause biphasic elevations of ROS and the second wave of ROS elevation causes persistent reduction in self-renewing capacity of mouse bone marrow HSC.9 Mitochondrial dysfunction, but not constant elevation of ROS, is implicated in age-
associated decline in HSC function.18 Since involvement of mtDNA remains unknown, investigating whether aged HSC have mtDNA damage would be of particular interest. Collectively, identifying molecular ‘scars’ left by low-dose X-rays on HSC would help provide a precise evaluation of the long-term detrimental effects by med- ical radiographic examination and also find common mechanisms that underlie hematopoietic aging and dis- ease.
References
1. Röntgen WC. On a New Kind of Rays. Science. 1896;3(59):227-231. 2. Daniel J. The X-Rays. Science. 1896;3(67):562-563.
3. Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ioniz-
ing radiation from medical imaging procedures. N Engl J Med.
2009;361(9):849-857.
4. Preston DL, Kusumi S, Tomonaga M, et al. Cancer incidence in
atomic bomb survivors. Part III. Leukemia, lymphoma and multiple
myeloma, 1950-1987. Radiat Res. 1994;137(2 Suppl):S68-97.
5. Hoeijmakers JH. Genome maintenance mechanisms for preventing
cancer. Nature. 2001;411(6835):366-374.
6. Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand
break repair in human cells exposed to very low x-ray doses. Proc
Natl Acad Sci U S A. 2003;100(9):5057-5062.
7. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT
scans in childhood and subsequent risk of leukaemia and brain
haematologica | 2020; 105(8)
1987