Page 191 - 2020_07-Haematologica-web
P. 191
Jagged1/2 and drug resistance in multiple myeloma
all the analyzed drugs, in agreement with the findings obtained in vitro.
To verify if the inhibitory approach based on J1/2KD had a translational potential, we recapitulated the experi- ments of MM-BMSC interplay by using IGOR1, a novel small molecule recently developed in our laboratory30 to uncouple Notch-Jagged interaction. IGOR1 is able to inhibit Notch activation in OPM2 cells and significantly increases the efficacy of the administered drugs, with a higher efficiency for Mel and Len (Figure 6B and C).
Jagged1 and Jagged2 blockade promotes sensitivity to bortezomib in a zebrafish xenograft myeloma model
Bortezomib is one of the most commonly used drugs for the treatment of newly-diagnosed and refractory MM patients.31 In recent years, several studies have supported the hypothesis that the development of resistance to such treatment is strongly dependent upon the BM microenvi- ronment, with a significant contribution of the CXCR4/SDF1α axis.32-34 Due to the results obtained in vitro concerning the role of this chemokine axis in the develop- ment of pharmacological resistance to Bor, we validated the effect of J1/2KD on MM cell resistance to Bor by tak- ing advantage of a novel zebrafish xenograft MM model.
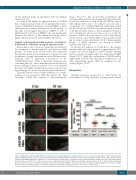
Zebrafish embryos were recently validated as a comple- mentary in vivo model for MM that allows the rapid screening of MM cells response to chemotherapeutic
drugs.35 Moreover, this model fully recapitulates the cytokine milieu present in the human BM, since zebrafish- secreted growth factors, such as IL6 and SDF1α, support MM cells growth in vivo.33,35 To validate our in vitro and ex vivo findings, Scr or J1/2KD U266 cells vitally labeled with the fluorescent dye CM-Dil were injected in the yolk area of 48 hpf zebrafish embryos. Xenotransplanted embryos were visualized by fluorescent microscopy to verify the presence of MM cells at the injection site at 2 hpi (Figure 7A-D), treated or not with 10 nM Bor and, visualized at 48 hpi for tumor cell growth (Figure 7A’-D’). Representative images of whole embryos are shown in Online Supplementary Figure S15.
As shown, the addition of 10 nM Bor to the embryo medium inhibited tumor growth of approximately 57% compared to controls (Figure 7A’ and B’), without affecting embryo viability. A similar effect was induced by J1/2KD (Figure 7A’-C’), while the combination of J1/2KD and Bor significantly reduced tumor growth in comparison to all other experimental groups (-82% in comparison to the control) (Figure 7A’-D’).
Discussion
Multiple myeloma progression is characterized by development of drug resistance causing patient relapse
Figure 7. Evaluation of tumor growth inhibition of myeloma cells xenotransplanted in zebrafish embryos. Fluorescent microscopy images of CM-Dil stained multiple myeloma (MM) xenografts at 2 hours (h) post injection (hpi) (A-D) and 48 h post injection (hpi) (A’-D’) into the yolk of zebrafish embryos. (A’-D’) Tumor growth analyses indicates that MM xenografts are responsive to treatment with bortezomib (Bor) (compare A’ and B’). Xenotransplanted J1/2KD cells also show reduced tumor growth (compare A’ and C’). These effects are increased combining the injection of J1/2KD cells with Bor treatment (compare A’, B’, C’ and D’). (E) Dot-plot shows the increase in tumor burden at 48 hpi, normalized to tumor area at 2 hpi (Scr+DMSO= 20 embryos; Scr+ Bor= 26 embryos; J1/2KD+ DMSO= 35 embryos; J1/2KD+ Bor=31 embryos). Statistical analysis was performed using one-way ANOVA and Tukey post-test (***P<0.001; ****P<0.0001).
haematologica | 2020; 105(7)
1933