Page 89 - Haematologica - Vol. 105 n. 6 - June 2020
P. 89
PRIMA-1Met and AZA combination in TP53-mutant MDS/AML
proteins.37 The SKM1 cell line has a p.R248Q mutation located in the DBD, while the other myeloid cell lines test- ed in this study have various TP53 truncation mutations that result in a lack of detectable p53 protein in all four cell lines.38,39 APR may not be able to restore an active confor- mation to the truncated p53 protein in these cell lines, thereby explaining the lower efficacy of APR compared with SKM1 cells. This also suggests that the effects of APR in these cell lines may be due in part to p53-independent processes.27 We then demonstrated that the inhibitory effect of the APR + AZA combination was synergistic in the five TP53-mutated cell lines that were tested,
A
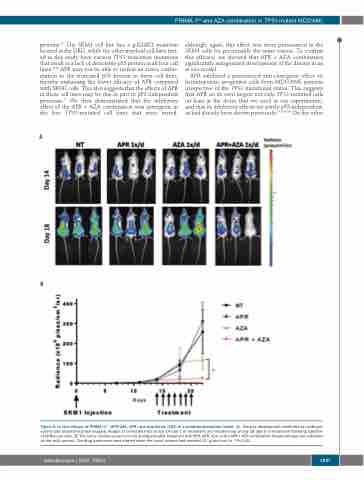
although, again, this effect was more pronounced in the SKM1 cells for presumably the same reason. To confirm this efficacy, we showed that APR + AZA combination significantly antagonized development of the disease in an in vivo model.
APR exhibited a pronounced anti-clonogenic effect on hematopoietic progenitor cells from MDS/AML patients irrespective of the TP53 mutational status. This suggests that APR on its own targets not only TP53-mutated cells (at least at the doses that we used in our experiments), and that its inhibitory effects are partly p53-independent, as had already been shown previously.27,34,35,40 On the other
B
Figure 5. In vivo efficacy of PRIMA-1Met (APR-246, APR) and azacitidine (AZA) in a xenotransplantation model. (A) Disease development monitored by luciferase activity and bioluminescence imaging. Images of untreated mice at day 14 (day 1 of treatment) and treated mice at day 18 (day 5 of treatment) following injection of SKM1-Luc cells. (B) The tumor volume (p/sec/cm2/sr) during and after treatment with PBS, APR, AZA, or the APR + AZA combination (treatment days are indicated by the solid arrows). The drug treatments were started when the tumor volume had reached 106 p/sec/cm2/sr. *P<0.05.
haematologica | 2020; 105(6)
1547